Abstract
Purpose
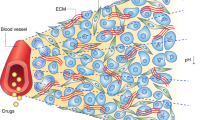
The intra-tumour distribution of anticancer drugs remains an important, but often under-estimated, influence on drug efficacy. Tumour acidity and the presence of efflux pumps were examined for their influence on the distribution of doxorubicin in a solid tumour model.
Methods
Anticancer drug distribution and overall accumulation was measured in tumour spheroids (TS) of varying sizes. The distribution profiles were examined in normoxic and hypoxic TS, the latter generating metabolic acidosis. Finally, the drug distribution profiles were related to efficacy using radial outgrowth assays.
Results
In large tumour spheroids (TS) (d ~500 μm), intracellular accumulation of doxorubicin was restricted to cells in the outermost layers and failed to accumulate within the viable cells in the ‘intermediate’ hypoxic zone. A similar profile was obtained for another protonatable amine, 7-AAD. In contrast, the distribution of the non-ionisable drug (at physiological pH) BODIPY-Taxol was uniform throughout the TS. In order to independently model the hypoxic and normoxic zones of TS, we compared drug accumulation in small entirely normoxic TS (d ~200 μm) with equivalent sized ones exposed to hypoxia in an anaerobic chamber. Exposure of TS to hypoxia caused a considerable reduction in the pH of the bathing medium and lower tissue accumulation of doxorubicin. Interstitial acidity reduces the proportion of doxorubicin in the non-ionised form.
Conclusions
In TS, the accumulation and distribution of doxorubicin was influenced by both the expression of P-glycoprotein and hypoxia-induced acidity. Therefore, optimisation of doxorubicin chemotherapy for hypoxic tumours will require circumvention of both of these crucial pharmacokinetic determinants.





Similar content being viewed by others
References
Baguley BC (2010) Multidrug resistance in cancer. Methods Mol Biol 596:1–14. doi:10.1007/978-1-60761-416-6_1
Gillet J-P, Gottesman MM (2010) Mechanisms of multidrug resistance in cancer. Methods Mol Biol 596:47–76. doi:10.1007/978-1-60761-416-6_4
Mellor HR, Callaghan R (2008) Resistance to chemotherapy in cancer: a complex and integrated cellular response. Pharmacology 81(4):275–300
Tredan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99(19):1441–1454. doi:djm135[pii]10.1093/jnci/djm135
Hietanen P, Blomqvist C, Wasenius VM, Niskanen E, Franssila K, Nordling S (1995) Do DNA ploidy and S-phase fraction in primary tumour predict the response to chemotherapy in metastatic breast cancer? Br J Cancer 71(5):1029–1032
Jackson RC (1989) The problem of the quiescent cancer cell. Adv Enzyme Regul 29:27–46
Siu WY, Arooz T, Poon RY (1999) Differential responses of proliferating versus quiescent cells to adriamycin. Exp Cell Res 250(1):131–141. doi:10.1006/excr.1999.4551S0014-4827(99)94551-2[pii]
Jiricny J (2006) The multifaceted mismatch-repair system. Nat Rev Mol Cell Biol 7(5):335–346
Rosell R, Taron M, Barnadas A, Scagliotti G, Sarries C, Roig B (2003) Nucleotide excision repair pathways involved in Cisplatin resistance in non-small-cell lung cancer. Cancer Control 10(4):297–305
Trivedi RN, Almeida KH, Fornsaglio JL, Schamus S, Sobol RW (2005) The role of base excision repair in the sensitivity and resistance to temozolomide-mediated cell death. Cancer Res 65(14):6394–6400
Deng X, Kornblau SM, Ruvolo PP, May WS Jr (2001) Regulation of Bcl2 phosphorylation and potential significance for leukemic cell chemoresistance. J Natl Cancer Inst Monogr 2000(28):30–37
Post LE (2002) Selectively replicating adenoviruses for cancer therapy: an update on clinical development. Curr Opin Investig Drugs 3(12):1768–1772
Sjostrom J, Blomqvist C, von Boguslawski K, Bengtsson NO, Mjaaland I, Malmstrom P, Ostenstadt B, Wist E, Valvere V, Takayama S, Reed JC, Saksela E (2002) The predictive value of bcl-2, bax, bcl-xL, bag-1, fas, and fasL for chemotherapy response in advanced breast cancer. Clin Cancer Res 8(3):811–816
Giaccone G, Gazdar AF, Beck H, Zunino F, Capranico G (1992) Multidrug sensitivity phenotype of human lung cancer cells associated with topoisomerase II expression. Cancer Res 52(7):1666–1674
Gokmen-Polar Y, Escuin D, Walls CD, Soule SE, Wang Y, Sanders KL, Lavallee TM, Wang M, Guenther BD, Giannakakou P, Sledge GW Jr (2005) Beta-tubulin mutations are associated with resistance to 2-methoxyestradiol in MDA-MB-435 cancer cells. Cancer Res 65(20):9406–9414
Hari M, Loganzo F, Annable T, Tan X, Musto S, Morilla DB, Nettles JH, Snyder JP, Greenberger LM (2006) Paclitaxel-resistant cells have a mutation in the paclitaxel-binding region of beta-tubulin (Asp26Glu) and less stable microtubules. Mol Cancer Ther 5(2):270–278
Matsumoto Y, Takano H, Nagao S, Fojo T (2001) Altered topoisomerase IIalpha and multidrug resistance-associated protein levels during drug selection: adaptations to increasing drug pressure. Jpn J Cancer Res 92(9):968–974
Desoize B, Jardillier J (2000) Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol 36(2–3):193–207. doi:S104084280000086X[pii]
Tannock IF, Lee CM, Tunggal JK, Cowan DS, Egorin MJ (2002) Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res 8(3):878–884
Konerding MA, Fait E, Gaumann A (2001) 3D microvascular architecture of pre-cancerous lesions and invasive carcinomas of the colon. Br J Cancer 84(10):1354–1362. doi:10.1054/bjoc.2001.1809S0007092001918099[pii]
Modok S, Hyde P, Mellor HR, Roose T, Callaghan R (2006) Diffusivity and distribution of vinblastine in three-dimensional tumour tissue: Experimental and mathematical modelling. Eur J Cancer 42:2404–2413
Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK (2004) Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64(11):3731–3736. doi:10.1158/0008-5472.CAN-04-007464/11/3731[pii]
Heldin CH, Rubin K, Pietras K, Ostman A (2004) High interstitial fluid pressure––an obstacle in cancer therapy. Nat Rev Cancer 4(10):806–813. doi:nrc1456[pii]10.1038/nrc1456
Patel KJ, Tannock IF (2009) The influence of P-glycoprotein expression and its inhibitors on the distribution of doxorubicin in breast tumors. BMC Cancer 9:356. doi:1471-2407-9-356[pii]10.1186/1471-2407-9-356
Primeau AJ, Rendon A, Hedley D, Lilge L, Tannock IF (2005) The distribution of the anticancer drug doxorubicin in relation to blood vessels in solid tumors. Clin Cancer Res 11(24):8782–8788. doi:11/24/8782[pii]10.1158/1078-0432.CCR-05-1664
Matherly LH (2001) Molecular and cellular biology of the human reduced folate carrier. Prog Nucleic Acid Res Mol Biol 67:131–162
Gorlick R, Goker E, Trippett T, Steinherz P, Elisseyeff Y, Mazumdar M, Flintoff WF, Bertino JR (1997) Defective transport is a common mechanism of acquired methotrexate resistance in acute lymphocytic leukemia and is associated with decreased reduced folate carrier expression. Blood 89(3):1013–1018
Ifergan I, Meller I, Issakov J, Assaraf YG (2003) Reduced folate carrier protein expression in osteosarcoma: implications for the prediction of tumor chemosensitivity. Cancer 98(9):1958–1966. doi:10.1002/cncr.11741
Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2(1):48–58
Modok S, Mellor HR, Callaghan R (2006) Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol 6(4):350–354
Keppler D, Konig J (2000) Hepatic secretion of conjugated drugs and endogenous substances. Semin Liver Dis 20(3):265–272
Loe DW, Almquist KC, Cole SP, Deeley RG (1996) ATP-dependent 17 beta-estradiol 17-(beta-D-glucuronide) transport by multidrug resistance protein (MRP). Inhibition by cholestatic steroids. J Biol Chem 271(16):9683–9689
Loe DW, Stewart RK, Massey TE, Deeley RG, Cole SP (1997) ATP-dependent transport of aflatoxin B1 and its glutathione conjugates by the product of the multidrug resistance protein (MRP) gene. Mol Pharmacol 51(6):1034–1041
Adams DJ (2005) The impact of tumor physiology on camptothecin-based drug development. Curr Med Chem Anticancer Agents 5(1):1–13
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324(5930):1029–1033. doi:324/5930/1029[pii]10.1126/science.1160809
Yeluri S, Madhok B, Prasad KR, Quirke P, Jayne DG (2009) Cancer’s craving for sugar: an opportunity for clinical exploitation. J Cancer Res Clin Oncol 135(7):867–877. doi:10.1007/s00432-009-0590-8
Warburg O (1956) On the origin of cancer cells. Science 123(3191):309–314
Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL (2006) HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291(5):L941–L949. doi:00528.2005[pii]10.1152/ajplung.00528.2005
Ullah MS, Davies AJ, Halestrap AP (2006) The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281(14):9030–9037. doi:M511397200[pii]10.1074/jbc.M511397200
Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL (2000) Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res 60(24):7075–7083
Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP (2002) Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res 62(12):3387–3394
Wartenberg M, Ling FC, Muschen M, Klein F, Acker H, Gassmann M, Petrat K, Putz V, Hescheler J, Sauer H (2003) Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J 17(3):503–505. doi:10.1096/fj.02-0358fje02-0358fje[pii]
Lee CM, Tannock IF (2006) Inhibition of endosomal sequestration of basic anticancer drugs: influence on cytotoxicity and tissue penetration. Br J Cancer 94(6):863–869. doi:6603010[pii]10.1038/sj.bjc.6603010
Mellor HR, Ferguson DJ, Callaghan R (2005) A model of quiescent tumour microregions for evaluating multicellular resistance to chemotherapeutic drugs. Br J Cancer 93(3):302–309
Mellor HR, Snelling S, Hall MD, Modok S, Jaffar M, Hambley TW, Callaghan R (2005) The influence of tumour microenvironmental factors on the efficacy of cisplatin and novel platinum (IV) complexes. Biochem Pharmacol 70(8):1137–1146
Martin C, Walker J, Rothnie A, Callaghan R (2003) The expression of P-glycoprotein does influence the distribution of novel fluorescent compounds in solid tumour models. Br J Cancer 89:1581–1589
Walker J, Martin C, Callaghan R (2004) Inhibition of P-glycoprotein function by XR9576 in a solid tumour model can restore anticancer drug efficacy. Eur J Cancer 40:594–605
De Milito A, Fais S (2005) Tumor acidity, chemoresistance and proton pump inhibitors. Future Oncol 1(6):779–786. doi:10.2217/14796694.1.6.779
Henning Ta, Kraus Mb, Brischwein Ma, AMa Otto, Ba Wolf (2004) Relevance of tumor microenvironment for progression, therapy and drug development. Anti-Cancer Drugs 15(1):7–14
Marie JP, Zhou DC, Gurbuxani S, Legrand O, Zittoun R (1996) MDR1/P-glycoprotein in haematological neoplasms. Eur J Cancer 32A(6):1034–1038
Trock BJ, Leonessa F, Clarke R (1997) Multidrug resistance in breast cancer: a meta-analysis of MDR1/gp170 expression and its possible functional significance. J Natl Cancer Inst 89(13):917–931
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo C, Han S, Liu J, Sun S, Han Z, Wu K, Fan D (2008) Hypoxia-inducible factor-1 alpha contributes to hypoxia-induced chemoresistance in gastric cancer. Cancer Sci 99(1):121–128. doi:CAS643[pii]10.1111/j.1349-7006.2007.00643.x
Fradette C, Batonga J, Teng S, Piquette-Miller M, du Souich P (2007) Animal models of acute moderate hypoxia are associated with a down-regulation of CYP1A1, 1A2, 2B4, 2C5, and 2C16 and up-regulation of CYP3A6 and P-glycoprotein in liver. Drug Metab Dispos 35(5):765–771. doi:dmd.106.013508[pii]10.1124/dmd.106.013508
Xiao-Dong L, Zhi-Hong Y, Hui-Wen Y (2008) Repetitive/temporal hypoxia increased P-glycoprotein expression in cultured rat brain microvascular endothelial cells in vitro. Neurosci Lett 432(3):184–187. doi:S0304-3940(07)01279-7[pii]10.1016/j.neulet.2007.12.017
Lotz C, Kelleher DK, Gassner B, Gekle M, Vaupel P, Thews O (2007) Role of the tumor microenvironment in the activity and expression of the p-glycoprotein in human colon carcinoma cells. Oncol Rep 17(1):239–244
Sauvant C, Nowak M, Wirth C, Schneider B, Riemann A, Gekle M, Thews O (2008) Acidosis induces multi-drug resistance in rat prostate cancer cells (AT1) in vitro and in vivo by increasing the activity of the p-glycoprotein via activation of p38. Int J Cancer 123(11):2532–2542. doi:10.1002/ijc.23818
Thews O, Gassner B, Kelleher DK, Gekle M (2008) Activity of drug efflux transporters in tumor cells under hypoxic conditions. Adv Exp Med Biol 614:157–164. doi:10.1007/978-0-387-74911-2_19
Netti PA, Hamberg LM, Babich JW, Kierstead D, Graham W, Hunter GJ, Wolf GL, Fischman A, Boucher Y, Jain RK (1999) Enhancement of fluid filtration across tumor vessels: implication for delivery of macromolecules. Proc Natl Acad Sci USA 96(6):3137–3142
Stohrer M, Boucher Y, Stangassinger M, Jain RK (2000) Oncotic pressure in solid tumors is elevated. Cancer Res 60(15):4251–4255
Swietach P, Patiar S, Supuran CT, Harris AL, Vaughan-Jones RD (2009) The role of carbonic anhydrase 9 in regulating extracellular and intracellular ph in three-dimensional tumor cell growths. J Biol Chem 284(30):20299–20310. doi:M109.006478[pii]10.1074/jbc.M109.006478
Glunde K, Düßmann H, Juretschke H-P, Leibfritz D (2002) Na < sup >+</sup >/H < sup >+</sup > exchange subtype 1 inhibition during extracellular acidification and hypoxia in glioma cells. J Neurochem 80(1):36–44
Whittingtons DA, Grubb JH, Waheed A, Shah GN, Sly WS, Christianson DW (2004) Expression, assay, and structure of the extracellular domain of murine carbonic anhydrase XIV. J Biol Chem 279(8):7223–7228. doi:10.1074/jbc.M310809200
Winum J-Y, Rami M, Scozzafava A, Montero J-L, Supuran C (2008) Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med Res Rev 28(3):445–463
Kaluz S, Kaluzová M, Liao S-Y, Lerman M, Stanbridge EJ (2009) Transcriptional control of the tumor- and hypoxia-marker carbonic anhydrase 9: a one transcription factor (HIF-1) show? Biochim Biophys Acta (BBA)––Rev Cancer 1795(2):162–172
Rodriguez-Enriquez S, Gallardo-Perez JC, Aviles-Salas A, Marin-Hernandez A, Carreno-Fuentes L, Maldonado-Lagunas V, Moreno-Sanchez R (2008) Energy metabolism transition in multi-cellular human tumor spheroids. J Cell Physiol 216(1):189–197. doi:10.1002/jcp.21392
Enerson BE, Drewes LR (2003) Molecular features, regulation, and function of monocarboxylate transporters: Implications for drug delivery. J Pharm Sci 92(8):1531–1544
Sonveaux P, Vegran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelley MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest 118(12):3930–3942. doi:36843[pii]10.1172/JCI36843
Semenza GL (2008) Tumor metabolism: cancer cells give and take lactate. J Clin Invest 118(12):3835–3837
Miraglia E, Viarisio D, Riganti C, Costamagna C, Ghigo D, Bosia A (2005) Na +/H + exchanger activity is increased in doxorubicin-resistant human colon cancer cells and its modulation modifies the sensitivity of the cells to doxorubicin. Int J Cancer 115(6):924–929
Acknowledgments
This research was funded by a Cancer Research UK Program grant (SP1861/0401) awarded to RC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mellor, H.R., Callaghan, R. Accumulation and distribution of doxorubicin in tumour spheroids: the influence of acidity and expression of P-glycoprotein. Cancer Chemother Pharmacol 68, 1179–1190 (2011). https://doi.org/10.1007/s00280-011-1598-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-011-1598-8