Abstract
Purpose
Corticorelin acetate (CrA) is a synthetic form of corticotropin-releasing factor undergoing clinical trials in the treatment of peritumoral brain edema (PBE). We sought to investigate preclinically its potential as an antitumor agent against human solid tumors and to assess its ability to enhance the therapeutic activity of bevacizumab (BEV) in these same models.
Methods
The in vivo efficacy of CrA as a single agent and in combination with the antiangiogenic agent, BEV, was examined in two preclinical human tumor models, the MX-1 breast and Colo-205 colon carcinomas. These models were selected based on their known sensitivity to BEV and were tumor types in which BEV has been approved for clinical use. The corneal micropocket assay was also performed to assess the antiangiogenic activity of CrA relative to BEV. The exposure level of CrA in the mouse using a typical preclinical regimen was measured so as to compare it to reported clinical exposure levels.
Results
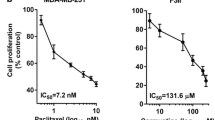
CrA was active as a single agent in the MX-1 breast carcinoma, but did not exhibit statistically significant activity as a single agent in the Colo-205 colon carcinoma under the doses and schedules used in the study. When BEV, which was active or near active in both the MX-1 and Colo-205 models, was administered concomitantly with CrA, therapeutic outcomes were observed that were significantly better than those obtained using either monotherapy. These therapeutic potentiations using CrA plus BEV were obtained in the absence of any observable increase in toxicities. CrA was active in the corneal micropocket assay, producing a substantial (>70%) inhibition of neovascularization. A representative CrA regimen in mice produced an exposure within eightfold of human exposure determined at one-half the current clinical dose.
Conclusions
The application of CrA for the treatment of PBE likely involves its activity as an antiangiogenic agent, which may be one possible mechanism to explain its observed preclinical antitumor activity. That activity, as well as its ability to provide an enhanced therapeutic outcome when given in conjunction with BEV in the absence of increased toxicity, supports the use of CrA clinically as other than a replacement therapy for dexamethasone in PBE.



Similar content being viewed by others
References
Stummer W (2007) Mechanisms of tumor-related brain edema. Neurosurg Focus 22:E8
Lee SW, Kim WJ, Park JA, Choi YK, Kwon YW, Kim KW (2006) Blood-brain barrier interfaces and brain tumors. Arch Pharm Res 29:265–275
Tjuvajev J, Uehara H, Desai R, Beattie B, Matei I, Zhou Y, Kreek MJ, Koutcher J, Blasberg R (1996) Corticotropin-releasing factor decreases vasogenic edema. Cancer Res 56:1352–1360
Kaal EC, Vecht CJ (2004) The management of brain edema in brain tumors. Curr Opin Oncol 16:593–600
Grammatopoulos DK, Chrousos GP (2002) Functional characteristics of CRH receptors and potential clinical applications of CRH-receptor antagonists. Trends Endocrinol Metab 13:436–444
Hillhouse EW, Grammatopoulos OK (2006) The molecular mechanisms underlying the regulation of the biological activity of corticotropin releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27:260–286
Reubi JC, Waser W, Vale W, Rivier J (2003) Expression of CRF1 and CRF2 receptors in human cancers. J Clin Endocrinol Metab 88:3312–3320
Bale TL, Giordano FJ, Vale WW (2003) A new role for corticotrophin-releasing factor receptor-2: suppression of vascularization. Trends Cardiovasc Med 13:68–71
Wang J, Xu Y, Xu Y, Zhu H, Zhang R, Zhang G, Li S (2008) Urocortin’s inhibition of tumor growth and angiogenesis in hepatocellular carcinoma via corticotrophin-releasing factor receptor 2. Cancer Invest 26:359–368
Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D’Amato RJ (1996) A model of angiogenesis in the mouse cornea. Invest Ophtalmol Vis Sci 37:1625–1632
Recht LD, Mechtler L, Phuphanich S, Hormigo A, Hines V, Milsted R, O’Connor PC, Ryan RP, Wong ET (2009) A placebo-controlled study investigating the dexamethasone-sparing effects of corticorelin acetate in patients with primary or metastatic brain tumors and peritumoral edema. J Clin Oncol 27:abstr 2078
Mechtler L, Wong ET, Hormigo A, Pannullo S, Hines V, Milsted R, O’Connor PO, Ryan RP, Recht L (2009) A long-term open-label extension study examining the steroid-sparing effects of corticorelin acetate in patients with cerebral tumors. J Clin Oncol 27:abstr 2079
Shapiro WR, Mechtler L, Cher L, Wheeler H, Hines V, Milsted R, O’Connor PC, Ryan RP, L. Recht L (2009) A randomized, double-blind study comparing corticorelin acetate with dexamethasone in patients with primary malignant glioma who require increased dexamethasone doses to control symptoms of peritumoral brain edema. J Clin Oncol 27:abstr 2080
Moliterno JA, Henry E, Pannullo SC (2009) Corticorelin acetate injections for the treatment of peritumoral brain edema. Expert Opin Investig Drugs 18:1413–1419
Ryan R, Moroz M, Berestein T, Evans-Freke S, Gamez I, Hines V, and Blasberg R (2009) Corticorelin acetate exhibits preclinical antitumor activity against the human glioma U87 xenograft. Proc Am Assoc Cancer Res, abstr 2326
Gamez I, Ryan R, and Keir S (2010) Corticorelin acetate exhibits preclinical antitumor activity, alone and with bevacizumab, against human brain tumor models. Proc Am Assoc Cancer Res, abstr 1557
Hsu JY, Wakelee HA (2009) Monoclonal antibodies targeting vascular endothelial growth factor: current status and future challenges in cancer therapy. BioDrugs 23(5):289–304
Kleinbaum DG, Klein M (2005) Survival analysis: a self-learning text, 2nd edn. Spring, Statistics for Biology and Health, New York
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice-Hall, Upper Saddle River
Higgins B, Kolinsky K, Linn M, Adames V, Zhang YE, Moisa C, Dugan U, Heimbrook D, Packman K (2007) Antitumor activity of capecitabine and bevacizumab combination in a human estrogen receptor-negative breast adenocarcinoma xenograft model. Anticancer Res 27:2279–2287
Yanagisawa M, Fujimoto-Ouchi K, Yorozu K, Yamashita Y, Mori K (2009) Antitumor activity of bevacizumab in combination with capecitabine and oxaliplatin in human colorectal cancer xenograft models. Oncol Rep 22:241–247
Gerber HP, Ferrara N (2005) Pharmacology and pharmacodynamics of bevacizumab as monotherapy or in combination with cytotoxic therapy in preclinical studies. Cancer Res 65:671–680
Warren RS, Yuan H, Matli MR, Gillett NA, Ferrara N (1995) Regulation by vascular endothelial growth factor of human colon cancer tumorigenesis in a mouse model of experimental liver metastasis. J Clin Invest 95:1789–1797
Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, Ferrara N (1993) Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 362:841–844
Mordenti J, Thomsen K, Licko V, Chen H, Meng YG, Ferrara N (1999) Efficacy and concentration-response of murine anti-VEGF monoclonal antibody in tumor-bearing mice and extrapolation to humans. Toxicol Pathol 27:14–21
Lee SY, Kim DK, Cho JH, Koh JY, Yoon YH (2008) Inhibitory effect of bevacizumab on the angiogenesis and growth of retinoblastoma. Arch Ophthalmol 126:953–958
Hao Z, Huang Y, Cleman J, Jovin IS, Vale WW, Bale TL, Giordano FJ (2008) Urocortin 2 inhibits tumor growth via effects on vascularization and cell proliferation. Proc Natl Acad Sci USA 105:3939–3944
Tjuvajev J, Kolesnikov Y, Joshi R, Sherinski J, Koutcher L, Zhou Y, Matei C, Koutcher J, Kreek MJ, Blasberg R (1998) Anti-neoplastic properties of human corticotropin releasing factor: involvement of the nitric oxide pathway. In Vivo 12:1–10
Investigator’s Brochure Xerecept® (Corticorelin acetate): Version 1.0 Celtic Pharma Development Services Inc., New York
Schulte HM, Chrousos GP, Gold PW, Booth JD, Oldfield EH, Cutler GB Jr, Loriaux DL (1985) Continuous administration of synthetic ovine corticotropin-releasing factor in man. Physiological and pathophysiological implications. J Clin Invest 75:1781–1785
Acknowledgments
The contributions of the research staffs from Piedmont Research Center and the Farmington Pharma Development Corporation in conjunction with the Hartford Hospital are duly recognized.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gamez, I., Ryan, R.P., Reid, L.D. et al. Corticorelin acetate, a synthetic corticotropin-releasing factor with preclinical antitumor activity, alone and with bevacizumab, against human solid tumor models. Cancer Chemother Pharmacol 67, 1415–1422 (2011). https://doi.org/10.1007/s00280-010-1437-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1437-3