Abstract
Purpose
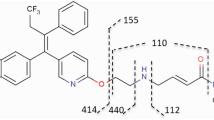
SR16157 is a novel dual-acting inhibitor of estrogen action that irreversibly inhibits the estrogen biosynthetic enzyme steroid sulfatase (STS) and releases the selective estrogen receptor modulator SR16137, which blocks the estrogen receptor. SR16157 is a promising agent for the endocrine therapy of breast cancer. We conducted preclinical in vivo toxicity evaluations to determine the maximum-tolerated dose (MTD), target organ(s) of toxicity, reversibility, dose-limiting toxicity, no observable adverse effect level (NOAEL), and toxicokinetics (TK) and to investigate a potential biomarker for use in SR16157 clinical trials.
Methods
SR16157 was administered to female Fischer 344 rats or beagle dogs by oral gavage (po) or capsule. Intravenous (iv) groups were included for the determination of bioavailability. Endpoints evaluated included clinical observations, body weights, hematology, serum chemistry, pharmacokinetics, TK, pathology of tissues, and STS activity in liver, or peripheral blood mononuclear cells (PBMCs).
Results
For rats, the MTD (i.e., the highest dose that did not cause lethality but produced toxicity) was 33 mg/kg/day (198 mg/m2/day), and the NOAEL was <10 mg/kg/day (60 mg/m2/day). For dogs, the MTD was estimated to exceed 10 mg/kg/day (200 mg/m2/day), and the NOAEL was estimated to be at or above 2.5 mg/kg/day (50 mg/m2/day).
Conclusions
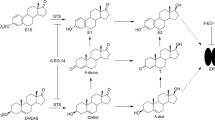
Our studies demonstrate that SR16157 has excellent pharmacokinetic properties and an acceptable toxicological profile. Modulation of STS activity in PBMCs appeared to be a possible biomarker for use in future clinical trials of SR16157.





Similar content being viewed by others
References
Janicke F (2004) Are all aromatase inhibitors the same? A review of the current evidence. Breast 13:S10–S18
Santner SJ, Feil PD, Santen RJ (1984) In situ estrogen production via the estrone sulfatase pathway in breast tumors: relative importance versus the aromatase pathway. J Clin Endocrinol Metab 59:29–33
Adams J, Garcia M, Rochefort H (1981) Estrogenic effects of physiological concentrations of 5-androstene-3 beta, 17 beta-diol and its metabolism in MCF7 human breast cancer cells. Cancer Res 4(11 Pt):4720–4726
Poulin R, Labrie F (1986) Stimulation of cell proliferation, estrogenic response by adrenal C19-delta 5-steroids in the ZR-73–1 human breast cancer cell line. Cancer Res 46(10):4933–4937
Maggiolini M, Donze O, Jeannin E, Ando S, Picard D (1999) Adrenal androgens stimulate the proliferation of breast cancer cells as direct activators of estrogen receptor. Cancer Res 59:4864–4869
Purohit A, Williams GJ, Howarth NM, Potter BV, Reed MJ (1995) Inactivation of steroid sulfatase by an active site-directed inhibitor, estrone-3-O-sulfamate. Biochemistry 34:11508–11514
Ciobanu LC, Boivin RP, Luu-The V, Poirier D (2001) Synthesis steroid sulphatase inhibitory activity of C19-and C21-steroidal derivatives bearing a benzyl-inhibiting group. Eur J Med Chem 36(7–8):659–671
Stanway SJ, Purohit A, Woo LW, Sufi S, Vigushin D, Ward R, Wilson RH, Stanczyk FZ, Dobbs N, Kulinskaya E, Elliott M, Potter BV, Reed MJ, Coombes RC (2006) Phase I study of STX 64 (667 Coumate) in breast cancer patients: the first study of a steroid sulfatase inhibitor. Clin Cancer Res 12(5):1585–1592
Zaveri N, Chao WR, Peters R, Tanabe M, Yamada Y, Toko T, Asao T, Eshima K (2003) A novel dual targeted approach for the endocrine therapy and prevention of breast cancer. SR16158, a novel steroid sulfatase inhibitor/tissue-selective antiestrogen SERM. Presented at American Association for Cancer Research Advances in Breast Cancer Symposium, Huntington Beach, CA
Rasmussen LM, Zaveri NT et al (2007) A novel dual-target steroid sulfatase inhibitor and antiestrogen: SR16157, a promising agent for the therapy of breast cancer. Breast Cancer Res Treat 106:191–203
Doppalapudi RS, Riccio ES, Rausch LL et al (2007) Evaluation of chemopreventative agents for genotoxic activity. Mutat Res 629:148–160
U.S. Federal Drug Administration (2003) FDA guidance for industry and reviewers estimating safe starting dose for therapeutics in adult healthy volunteers, FR vol 68, pp 2340–2341, 16 Jan 2003. Accessed 10 Oct 2009 from http://www.fda.gov/cber/gdlns/dose.htm
Acknowledgments
This work was supported under Contract N01-CM-42203 issued by the Developmental Therapeutics Program, Division of Cancer Treatment and Diagnosis, NCI. The preclinical development of SR16157 was sponsored by NCI’s Rapid Access to Intervention Development Program, Cycles 9 and 13, PI: Zaveri; the authors would also like to thank Wan-Ru Chao and Mas Tanabe for their MCF-7 xenograft efficacy work in nude mice.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rausch, L., Green, C., Steinmetz, K. et al. Preclinical pharmacokinetic, toxicological and biomarker evaluation of SR16157, a novel dual-acting steroid sulfatase inhibitor and selective estrogen receptor modulator. Cancer Chemother Pharmacol 67, 1341–1352 (2011). https://doi.org/10.1007/s00280-010-1430-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1430-x