Abstract
Purpose
PR-104, a bioreductive prodrug in clinical trial, is a phosphate ester which is rapidly metabolized to the corresponding alcohol PR-104A. This dinitrobenzamide mustard is activated by reduction to hydroxylamine (PR-104H) and amine (PR-104M) metabolites selectively in hypoxic cells, and also independently of hypoxia by aldo-keto reductase (AKR) 1C3 in some tumors. Here, we evaluate reductive metabolism of PR-104A in mice and its significance for host toxicity.
Methods
The pharmacokinetics of PR-104, PR-104A and its reduced metabolites were investigated in plasma and tissues of mice (with and without SiHa or H460 tumor xenografts) and effects of potential oxidoreductase inhibitors were evaluated.
Results
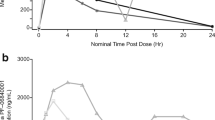
Pharmacokinetic studies identified extensive non-tumor reduction of PR-104A to the 5-amine PR-104H (identity of which was confirmed by chemical synthesis), especially in liver. However, high concentrations of PR-104H in tumors that suggested intra-tumor activation is also significant. The tissue distribution of PR-104M/H was broadly consistent with the target organ toxicities of PR-104 (bone marrow, intestines and liver). Surprisingly, hepatic nitroreduction was not enhanced when the liver was made more hypoxic by hepatic artery ligation or breathing of 10% oxygen. A screen of non-steroidal anti-inflammatory drugs identified naproxen as an effective AKR1C3 inhibitor in human tumor cell cultures and xenografts, suggesting its potential use to ameliorate PR-104 toxicity in patients. However, neither naproxen nor the pan-CYP inhibitor 1-aminobenzotriazole inhibited normal tissue reduction of PR-104A in mice.
Conclusions
PR-104 is extensively reduced in mouse tissues, apparently via oxygen-independent two-electron reduction, with a tissue distribution that broadly reflects toxicity.





Similar content being viewed by others
References
Workman P, Stratford IJ (1993) The experimental development of bioreductive drugs and their role in cancer therapy. Cancer Metastasis Rev 12:73–82
Wardman P (2001) Electron transfer and oxidative stress as key factors in the design of drugs selectively active in hypoxia. Curr Med Chem 8:739–761
Brown JM, Wilson WR (2004) Exploiting tumor hypoxia in cancer treatment. Nat Rev Cancer 4:437–447
McKeown SR, Cowen RL, Williams KJ (2007) Bioreductive drugs: from concept to clinic. Clin Oncol 19:427–442
Chen Y, Hu L (2009) Design of anticancer prodrugs for reductive activation. Med Res Rev 29:29–64
Mason RP, Holtzman JL (1975) The role of catalytic superoxide formation in the O2 inhibition of nitroreductase. Biochem Biophys Res Commun 67:1267–1274
Siim BG, Wilson WR (1995) Efficient redox cycling of nitroquinoline bioreductive drugs due to aerobic nitroreduction in Chinese hamster cells. Biochem Pharmacol 50:75–82
Vaupel P, Schlenger K, Knoop C, Hockel M (1991) Oxygenation of human tumors: evaluation of tissue oxygen distribution in breast cancers by computerized O2 tension measurements. Cancer Res 51:3316–3322
Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, Hill RP, Koch CJ, Krishna MC, Krohn KA, Lewis JS, Mason RP, Melillo G, Padhani AR, Powis G, Rajendran JG, Reba R, Robinson SP, Semenza GL, Swartz HM, Vaupel P, Yang D, Croft B, Hoffman J, Liu G, Stone H, Sullivan D (2006) Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 82:699–757
Lunt SJ, Chaudary N, Hill RP (2009) The tumor microenvironment and metastatic disease. Clin Exp Metastasis 26:19–34
Skelly JV, Knox RJ, Jenkins TC (2001) Aerobic nitroreduction by flavoproteins: enzyme structure, mechanisms and role in cancer chemotherapy. Mini Rev Med Chem 1:293–306
Danson S, Ward TH, Butler J, Ranson M (2004) DT-diaphorase: a target for new anticancer drugs. Cancer Treat Rev 30:437–449
Walton MI, Wolf CR, Workman P (1989) Molecular enzymology of the reductive bioactivation of hypoxic cell cytotoxins. Int J Radiat Oncol Biol Phys 16:983–986
Patterson AV, Ferry DM, Edmunds SJ, Gu Y, Singleton RS, Patel K, Pullen SM, Syddall SP, Atwell GJ, Yang S, Denny WA, Wilson WR (2007) Mechanism of action and preclinical antitumor activity of the novel hypoxia-activated DNA crosslinking agent PR-104. Clin Cancer Res 13:3922–3932
Jameson MB, Rischin D, Pegram M, Gutheil J, Patterson AV, Denny WA, Wilson WR (2010) A phase I pharmacokinetic trial of PR-104, a nitrogen mustard prodrug activated by both hypoxia and aldo-keto reductase 1C3, in patients with solid tumors. Cancer Chemother Pharmacol 65:791–801
Patel K, Lewiston D, Gu Y, Hicks KO, Wilson WR (2007) Analysis of the hypoxia-activated dinitrobenzamide mustard phosphate prodrug PR-104 and its alcohol metabolite PR-104A in plasma and tissues by liquid chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 856:302–311
Singleton RS, Guise CP, Ferry DM, Pullen SM, Dorie MJ, Brown JM, Patterson AV, Wilson WR (2009) DNA crosslinks in human tumor cells exposed to the prodrug PR-104A: relationships to hypoxia, bioreductive metabolism and cytotoxicity. Cancer Res 69:3884–3891
Helsby NA, Wheeler SJ, Pruijn FB, Palmer BD, Yang S, Denny WA, Wilson WR (2003) Effect of nitroreduction on the alkylating reactivity and cytotoxicity of the 2,4-dinitrobenzamide-5-aziridine CB 1954 and the corresponding nitrogen mustard SN 23862: distinct mechanisms of bioreductive activation. Chem Res Toxicol 16:469–478
Gu Y, Patterson AV, Atwell GJ, Chernikova SB, Brown JM, Thompson LH, Wilson WR (2009) Roles of DNA repair and reductase activity in the cytotoxicity of the hypoxia-activated dinitrobenzamide mustard PR-104A. Mol Cancer Ther 8:1714–1723
Guise CP, Wang A, Thiel A, Bridewell D, Wilson WR, Patterson AV (2007) Identification of human reductases that activate the dinitrobenzamide mustard prodrug PR-104A: a role for NADPH:cytochrome P450 oxidoreductase under hypoxia. Biochem Pharmacol 74:810–820
Guise CP, Abbattista M, Singleton RS, Holford SD, Connolly J, Dachs GU, Fox SB, Pollock R, Harvey J, Guilford P, Doñate F, Wilson WR, Patterson AV (2010) The bioreductive prodrug PR-104A is activated under aerobic conditions by human aldo-keto reductase 1C3. Cancer Res 70:1573–1584
Penning TM, Byrns MC (2009) Steroid hormone transforming aldo-keto reductases and cancer. Ann N Y Acad Sci 1155:33–42
Rizner TL, Smuc T, Rupreht R, Sinkovec J, Penning TM (2006) AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol Cell Endocrinol 248:126–135
Penning TM, Burczynski ME, Jez JM, Hung CF, Lin HK, Ma H, Moore M, Palackal N, Ratnam K (2000) Human 3alpha-hydroxysteroid dehydrogenase isoforms (AKR1C1–AKR1C4) of the aldo-keto reductase superfamily: functional plasticity and tissue distribution reveals roles in the inactivation and formation of male and female sex hormones. Biochem J 351:67–77
Jin Y, Penning TM (2007) Aldo-keto reductases and bioactivation/detoxication. Annu Rev Pharmacol Toxicol 47:263–292
Birtwistle J, Hayden RE, Khanim FL, Green RM, Pearce C, Davies NJ, Wake N, Schrewe H, Ride JP, Chipman JK, Bunce CM (2009) The aldo-keto reductase AKR1C3 contributes to 7,12-dimethylbenz(a)anthracene-3,4-dihydrodiol mediated oxidative DNA damage in myeloid cells: implications for leukemogenesis. Mutat Res 662:67–74
Patel K, Holford N, Choy S, Hicks KO, Wilson WR (2010) A compartmental population pharmacokinetic model for the hypoxia-targeted dinitrobenzamide mustard prodrug PR-104 in humans, dogs, rats and mice. Cancer Chemother Pharmacol (submitted)
Gu Y, Wilson WR (2009) Rapid and sensitive ultra-high-pressure liquid chromatography–tandem mass spectrometry analysis of the novel anticancer agent PR-104 and its major metabolites in human plasma: application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci 877:3181–3186
Gu Y, Atwell GJ, Wilson WR (2010) Metabolism and excretion of the novel bioreductive prodrug PR-104 in mice, rats, dogs and humans. Drug Metab Dispos 38:498–508
Denny WA, Atwell GJ, Yang S, Wilson WR, Patterson AV, Helsby NA (2005) Novel nitrophenyl mustard and nitrophenylaziridine alcohols and their corresponding phosphates and their use as targeted cytotoxic agents.PCT/NZ 2004/529249
Atwell GJ, Denny WA (2007) Synthesis of 3H- and 2H4-labelled versions of the hypoxia-activated pre-prodrug 2-[(2-bromoethyl)-2,4-dinitro-6-[[[2-(phosphonooxy)ethyl]amino]carbonyl]anilino]ethyl methanesulfonate (PR-104). J Label Comp Radiopharm 50:7–12
(1988) UKCCCR guidelines for the welfare of animals in experimental neoplasia. Br J Cancer 58:109-113
Plock J, Frese S, Keogh A, Bisch-Knaden S, Ayuni E, Corazza N, Weikert C, Jakob S, Erni D, Dufour JF, Brunner T, Candinas D, Stroka D (2007) Activation of non-ischemic, hypoxia-inducible signalling pathways up-regulate cytoprotective genes in the murine liver. J Hepatol 47:538–545
Arteel GE, Thurman RG, Yates JM, Raleigh JA (1995) Evidence that hypoxia markers detect oxygen gradients in liver: pimonidazole and retrograde perfusion of rat liver. Br J Cancer 72:889–895
Gobec S, Brozic P, Rizner TL (2005) Nonsteroidal anti-inflammatory drugs and their analogues as inhibitors of aldo-keto reductase AKR1C3: new lead compounds for the development of anticancer agents. Bioorg Med Chem Lett 15:5170–5175
Byrns MC, Steckelbroeck S, Penning TM (2008) An indomethacin analogue, N-(4-chlorobenzoyl)-melatonin, is a selective inhibitor of aldo-keto reductase 1C3 (type 2 3alpha-HSD, type 5 17beta-HSD, and prostaglandin F synthase), a potential target for the treatment of hormone dependent and hormone independent malignancies. Biochem Pharmacol 75:484–493
Ortiz de Montellano PR, Mathews JM (1981) Autocatalytic alkylation of the cytochrome P-450 prosthetic haem group by 1-aminobenzotriazole. Isolation of an NN-bridged benzyne-protoporphyrin IX adduct. Biochem J 195:761–764
Balani SK, Li P, Nguyen J, Cardoza K, Zeng H, Mu DX, Wu JT, Gan LS, Lee FW (2004) Effective dosing regimen of 1-aminobenzotriazole for inhibition of antipyrine clearance in guinea pigs and mice using serial sampling. Drug Metab Dispos 32:1092–1095
Denny WA, Wilson WR (1986) Considerations for the design of nitrophenyl mustards as agents with selective toxicity for hypoxic tumor cells. J Med Chem 29:879–887
Zhang J, Tian Q, Yung CS, Chuen LS, Zhou S, Duan W, Zhu YZ (2005) Metabolism and transport of oxazaphosphorines and the clinical implications. Drug Metab Rev 37:611–703
Vanderkooi JM, Erecinska M, Silver IA (1991) Oxygen in mammalian tissue: methods of measurement and affinities of various reactions. Am J Physiol 260:C1131–C1150
Parmar K, Mauch P, Vergilio J, Sackstein R, Down JD (2007) Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 104:5431–5436
Hicks KO, Myint H, Patterson AV, Pruijn FB, Siim BG, Patel K, Wilson WR (2007) Oxygen dependence and extravascular transport of hypoxia-activated prodrugs: comparison of the dinitrobenzamide mustard PR-104A and tirapazamine. Int J Radiat Oncol Biol Phys 69:560–571
Velica P, Davies NJ, Rocha PP, Schrewe H, Ride JP, Bunce CM (2009) Lack of functional and expression homology between human and mouse aldo-keto reductase 1C enzymes: implications for modelling human cancers. Mol Cancer 8:121–132
Acknowledgments
This work was supported by the Health Research Council of New Zealand [Grant 08/103] and a Technology In Industry Fellowship to Y. Gu from the Foundation for Research, Science and Technology, New Zealand.
Conflict of interest statement
William R. Wilson is a founding scientist, stockholder and consultant to Proacta, Inc. Adam V. Patterson is a consultant to Proacta, Inc.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s00280-011-1597-9
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gu, Y., Guise, C.P., Patel, K. et al. Reductive metabolism of the dinitrobenzamide mustard anticancer prodrug PR-104 in mice. Cancer Chemother Pharmacol 67, 543–555 (2011). https://doi.org/10.1007/s00280-010-1354-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1354-5