Abstract
Purpose
To gain a better understanding of the incidence and risk of hypokalemia in patients who received cetuximab-based therapy.
Patients and methods
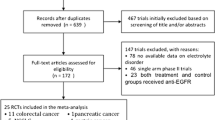
Databases, including Pubmed, EMBASE, The Cochrane Library, annual meeting of American Society of Clinical Oncology (2000–2008), and Web of science were searched to identify relevant studies. Eligible studies were prospective phase II–III clinical trials of patients with cancer assigned cetuximab at the dose of 400 mg/m2 IV on day 1 and 250 mg/m2 weekly thereafter. The primary endpoint was incidence of hypokalemia.
Results
Eleven clinical reports were identified which included a total of 2,254 patients who were available for analysis, with 1,324 patients assigned cetuximab-based treatment. The results showed high incidence of grade 3 and 4 hypokalemia [6.2% (95% CI 4.9–7.7)] and high incidence of all-grade hypokalemia [8.0% (95% CI 4.5–13.9)] associated with cetuximab-based therapy for advanced cancer. Compared with non-cetuximab therapy, cetuximab-based therapy has higher risk of grade 3 and 4 hypokalemia [1.81 (95% CI 1.12–2.93)].
Conclusion
Cetuximab-based therapy is associated with a significant risk of hypokalemia. Early monitoring and effective management of hypokalemia is important for patients that received cetuximab-based therapy.




Similar content being viewed by others
References
Baselga J (2001) The EGFR as a target for anticancer therapy—focus on cetuximab. Eur J Cancer 37(Suppl 4):S16–S22
Kim ES, Khuri FR, Herbst RS (2001) Epidermal growth factor receptor biology (IMC-C225). Curr Opin Oncol 13(6):506–513
Raymond E, Faivre S, Armand JP (2000) Epidermal growth factor receptor tyrosine kinase as a target for anticancer therapy. Drugs 60(Suppl 1):15–23 (discussion 41–42)
Ciardiello F, Tortora G (2002) Anti-epidermal growth factor receptor drugs in cancer therapy. Expert Opin Investig Drugs 11:755–768
Ciardiello F (2000) Epidermal growth factor receptor tyrosine kinase inhibitors as anticancer agents. Drugs 60(Suppl 1):25–32 (discussion 41–42)
Liu L, Cao Y, Tan A, Liao C, Gao F (2009) Cetuximab-based therapy versus non-cetuximab therapy for advanced cancer: a meta-analysis of 17 randomized controlled trials. Cancer Chemother Pharmacol [Epub ahead of print]
Saltz LB, Meropol NJ, Loehrer PJ Sr, Needle MN, Kopit J, Mayer RJ (2004) Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol 22:1201–1208
Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A et al (2004) Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 351:337–345
Burtness B, Goldwasser MA, Flood W, Mattar B, Forastiere AA, Eastern Cooperative Oncology Group (2005) Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J Clin Oncol 23(864):6–8654
Lin SH, Halperin ML (2007) Hypokalemia: a practical approach to diagnosis and its genetic basis. Curr Med Chem 14:1551–1565
Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ (1996) Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17(1):1–12
US National Cancer Institute. Common terminology criteria for adverse events v3.0(CTCAE). http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf
Pfister DG, Su YB, Kraus DH, Wolden SL, Lis E, Aliff TB, Zahalsky AJ, Lake S, Needle MN, Shaha AR, Shah JP, Zelefsky MJ (2006) Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase II study of a new combined-modality paradigm. J Clin Oncol 24(7):1072–1078
Cartwright TH, Kuefler P, Cohn A, Hyman W, Boehm KA, Ilegbodu D, Asmar L (2006) Results of a phase II trial of cetuximab + XELIRI as first-line therapy of patients with advanced and/or metastatic colorectal cancer. J Clin Oncol 24(18S):13502
Bennouna J, Faroux R, François E, Ligeza C, El Hannani C, Perrier H, Jacob J, Desseigne F, Perrocheau G, Douillard JY (2007) CETUFTIRI, a new combination of UFT with leucovorin (LV), irinotecan, and cetuximab as first-line treatment for patients (pts) with unresectable metastatic colorectal cancer (mCRC): preliminary results from a multicenter phase II trial. J Clin Oncol 25(18S):4087
Birnbaum AE, Johnson TT, Rathore R, Khurshid H, Puthawala M, Radie Keane K, Ruhl C, Wanebo H, Kennedy T, Ready N, BrUOG (2007) Induction cetuximab (C) followed by C, paclitaxel (P), carboplatin (CP) and concurrent radiation (RT) for locoregionally advanced squamous cell carcinoma of the head and neck (SCCHN). In: Annual meeting proceedings part I. (20 June Supplement). J Clin Oncol 25(18S):16504
Belani CP, Schreeder MT, Steis RG, Guidice RA, Marsland TA, Butler EH, Ramalingam SS (2008) Cetuximab in combination with carboplatin and docetaxel for patients with metastatic or advanced-stage nonsmall cell lung cancer: a multicenter phase 2 study. Cancer 113:2512–2517
Konner J, Schilder RJ, DeRosa FA, Gerst SR, Tew WP, Sabbatini PJ, Hensley ML, Spriggs DR, Aghajanian CA (2008) A phase II study of cetuximab/paclitaxel/carboplatin for the initial treatment of advanced-stage ovarian, primary peritoneal, or fallopian tube cancer. Gynecol Oncol 110:140–145
Pinto C, Di Fabio F, Barone C, Siena S, Falcone A, Rojas Llimpe FL, Cascinu S, Giaquinta S, Schinzari G, Mutri V, Martoni AA, BrUOG (2008) Cetuximab in combination with cisplatin and docetaxel as first-line treatment in patients with locally advanced or metastatic gastric or gastroesophageal junction (GEJ) adenocarcinoma (Italian phase II DOCETUX study). In: ASCO annual meeting proceedings part I. (20 June Supplement). J Clin Oncol 25(18S):16504
Safran H, Suntharalingam M, Dipetrillo T, Ng T, Doyle LA, Krasna M, Plette A, Evans D, Wanebo H, Akerman P, Spector J, Kennedy N, Kennedy T (2008) Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys 70:391–395
Sobrero AF, Maurel J, Fehrenbacher L, Scheithauer W, Abubakr YA, Lutz MP, Vega-Villegas ME, Eng C, Steinhauer EU, Prausova J, Lenz HJ, Borg C, Middleton G, Kröning H, Luppi G, Kisker O, Zubel A, Langer C, Kopit J, Burris HA III (2008) EPIC: phase III trial of cetuximab plus irinotecan after fluoropyrimidine and oxaliplatin failure in patients with metastatic colorectal cancer. J Clin Oncol 26:2311–2319
Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, Erfan J, Zabolotnyy D, Kienzer HR, Cupissol D, Peyrade F, Benasso M, Vynnychenko I, De Raucourt D, Bokemeyer C, Schueler A, Amellal N, Hitt R (2008) Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116–11127
Kokot F, Hyla-Klekot L (2008) Drug-induced abnormalities of potassium metabolism. Pol Arch Med Wewn 118:431–434
Ben Salem C, Hmouda H, Bouraoui K (2009) Drug-induced hypokalaemia. Curr Drug Saf 4:55–61
Chubanov V, Waldegger S, Mederos y Schnitzler M, Vitzthum H, Sassen MC, Seyberth HW, Konrad M, Gudermann T (2004) Disruption of TRPM6/TRPM7 complex formation by a mutation in the TRPM6 gene causes hypomagnesemia with secondary hypocalcemia. Proc Natl Acad Sci USA 101(9):2894–2899
Groenestege WM, Thebault S, Van Der Wijst J et al (2007) Impaired basolateral sorting of pro-EGF causes isolated recessive renal hypomagnesemia. J Clin Invest 117:2260–2267
Whang R, Welt LA (1963) Observations in experimental magnesium depletion. J Clin Invest 42:305–313
Hoskote SS, Joshi SR, Ghosh AK (2008) Disorders of potassium homeostasis: pathophysiology and management. J Assoc Physicians India 56:685–693
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cao, Y., Liu, L., Liao, C. et al. Meta-analysis of incidence and risk of hypokalemia with cetuximab-based therapy for advanced cancer. Cancer Chemother Pharmacol 66, 37–42 (2010). https://doi.org/10.1007/s00280-009-1131-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-1131-5