Abstract
Purpose
Preclinical data suggested that bryostatin-1 (bryo) could potentiate the cytotoxicity of cisplatin when given prior to this drug. We designed a phase I study to achieve tolerable doses and schedules of bryo and cisplatin in combination and in this sequence.
Methods
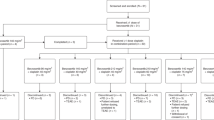
Patients with non-hematologic malignancies received bryo followed by cisplatin in several schedules. Bryo was given as an 1 and a 24 h continuous infusion, while cisplatin was always given over 1 h at 50 and 75 mg/m2; the combined regimen was repeated on an every 3-week and later on an every 2-week schedule. Bryo doses were escalated until recommended phase II doses were defined for each schedule. Patients were evaluated with computerized tomography every 2 cycles.
Results
Fifty-three patients were entered. In an every 2-week schedule, the 1-h infusion of bryo became limited by myalgia that was clearly cumulative. With cisplatin 50 mg/m2 its recommended phase II dose was 30 μg/m2. In the 3-week schedule, dose-limiting toxicities were mostly related to cisplatin effects while myalgias were tolerable. Pharmacokinetics unfortunately proved to be unreliable due to bryo’s erratic extraction. Consistent inhibition of PKC isoform eta (η) in peripheral blood mononuclear cells was observed following bryo.
Conclusions
Bryo can be safely administered with cisplatin with minimal toxicity; however, only four patients achieved an objective response. Modulation of cisplatin cytotoxicity by bryo awaits further insight into the molecular pathways involved.

Similar content being viewed by others
References
Aeder SE, Martin PM, Soh JW, Hussaini IM (2004) PKC-η mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene 23:9062–9069
Basu A, Lazo JS (1992) Sensitization of human cervical carcinoma cells to cis-diamminedichloroplatinum (II) by bryostatin 1. Cancer Res 52:3119–3124
Corbett AH, Fernald AW, Osheroff N (1993) Protein kinase C modulates the catalytic activity of topoisomerase II by enhancing the rate of ATP hydrolysis: evidence for a common mechanism of regulation by phosphorylation. Biochemistry 32:2090–2097
Dale IL, Bradshaw TD, Gescher A, Pettit GR (1989) Comparison of effects of bryostatins 1 and 2 and 12–O-tetradecanoylphorbol-13-acetate on protein kinase C activity in A549 human lung carcinoma cells. Cancer Res 49:3242–3245
El-Rayes BF, Gadgeel S, Shields AF, Manza S, Lorusso P, Philip PA (2006) Phase I study of bryostatin 1 and gemcitabine. Clin Cancer Res 12:7059–7062
Ford JM, Hait WN (1990) Pharmacology of drugs that alter multidrug resistance in cancer. Pharmacol Rev 42:155–199
Goodnight JE Jr, Moseley HS, Eilber FR, Sarna G, Morton DL (1979) Cis-dichlorodiammineplatinum (II) alone and combined with DTIC for treatment of disseminated malignant melanoma. Cancer Treat Rep 63:2005–2007
Grunicke H, Hofmann J, Maly K, Goddard PM, Kelland LR, Morgan SE (1991) Enhancement of the antiproliferative effect of cis-diammine-dichloroplatinum (II) and other antitumor agents by inhibition of enzymes involved in growth factor signal transduction. In: Howell SB (ed) Platinum and other metal coordination compounds in cancer chemotherapy. Plenum Press, New York, pp 161–172
Grunicke H, Hofmann J, Utz I, Uberall F (1994) Role of protein kinases in antitumor drug resistance. Ann Hematol 69:S1–S6
Grunicke HH, Uberall F (1992) Protein kinase C modulation. Semin Cancer Biol 3:351–360
Gschwendt M, Furstenberger G, Rose-John S, Rogers M, Kittstein W, Pettit GR, Herald CL, Marks F (1988) Bryostatin 1, an activator of protein kinase C, mimics as well as inhibits biological effects of the phorbol ester TPA in vivo and in vitro. Carcinogenesis 9:555–562
Gschwendt M, Leibersperger H, Kittstein W, Marks F (1992) Protein kinase C zeta and eta in murine epidermis. TPA induces down-regulation of PKC eta but not PKC zeta. FEBS Lett 307:151–155
Hallahan DE, Virudachalam S, Sherman ML, Huberman E, Kufe DW, Weichselbaum RR (1991) Tumor necrosis factor gene expression is mediated by protein kinase C following activation by ionizing radiation. Cancer Res 51:4565–4569
Hickman PF, Kemp GJ, Thompson CH, Salisbury AJ, Wade K, Harris AL, Radda GK (1995) Bryostatin 1, a novel antineoplastic agent and protein kinase C activator, induces human myalgia and muscle metabolic defects: a 31P magnetic spectroscopic study. Br J Cancer 72:998–1003
Hochster H, Liebes L, Speyer J, Sorich J, Taubes B, Oratz R, Wernz J, Chachoua A, Raphael B, Vinci RZ (1994) Phase I trial of low-dose continuous topotecan infusion in patients with cancer: an active and well-tolerated regimen. J Clin Oncol 12:553–559
Hofmann J, Doppler W, Jakob A, Maly K, Posch L, Uberall F, Grunicke HH (1988) Enhancement of the antiproliferative effect of cis-diamminedichloroplatinum (II) and nitrogen mustard by inhibitors of protein kinase C. Int J Cancer 42:382–388
Hornung RL, Pearson JW, Beckwith M, Longo DL (1992) Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in vitro versus in vivo activity. Cancer Res 52:101–107
Isonishi S, Andrews PA, Howell SB (1990) Increased sensitivity to cis-diamminedichloroplatinum (II) in human ovarian carcinoma cells in response to treatment with 12-O-tetradecanoylphorbol 13-acetate. J Biol Chem 265:3623–3627
Jackson DN, Foster DA (2004) The enigmatic protein kinase C delta: complex roles in cell proliferation and survival. FASEB J 18:627–636
Jayson GC, Crowther D, Prendiville J, McGown AT, Schneid C, Stern P, Young R, Brenchley P, Chang J, Owens S et al (1995) A Phase I trial of bryostatin 1 in patients with advanced malignancy using a 24 hour intravenous infusion. Br J Cancer 72:461–468
Liebes L, Potmesil M, Kim T, Pease D, Buckley M, Fry D, Cho J, Adler H, Dar K, Zeleniuch-Jacquotte A, Hochster H (1998) Pharmacodynamics of topoisomerase I inhibition: Western blot determination of topoisomerase I and cleavable complex in patients with upper gastrointestinal malignancies treated with topotecan. Clin Can Res 4:545–557
Masso-Welch PA, Winston JS, Edge S, Darcy KM, Asch H, Vaughan MM, Ip MM (2001) Altered expression and localization of PKC eta in human breast tumors. Breast Cancer Res Treat 68:211–223
Mohammad RM, Diwakaran H, Maki A, Emara MA, Pettit GR, Redman B, AL-Katib A (1995) Bryostatin 1 induces apoptosis and augments inhibitory effects of vincristine in human diffuse large cell lymphoma. Leuk Res 19:667–673
Nezhat F, Wadler S, Muggia F, Mandeli J, Goldberg G, Rahaman J, Runowicz C, Murgo AJ, Gardner GJ (2004) Phase II trial of the combination of bryostatin-1 and cisplatin in advanced or recurrent carcinoma of the cervix: a New York Gynecologic Oncology Group study. Gynecol Oncol 93:144–148
Osada SI, Hashimoto Y, Nomura S, Kohno Y, Chida K, Tajima O, Kubo K, Akimoto K, Koizumi H, Kitamura Y, Suzuki K, Ohno S, Kuroki T (1993) Predominant expression of nPKCη, a Ca2+-independent isoform of protein kinase C in epithelial tissues, in association with epithelial differentiation. Cell Growth Diff 4:167–175
Perego P, Casati G, Gambetta RA, Soranzo C, Zunino F (1993) Effect of modulation of protein kinase C activity on cisplatin cytotoxicity in cisplatin-resistant and cisplatin-sensitive human osteosarcoma cells. Cancer Lett 72:53–58
Pettit GR, Herald CL, Doubek DL, Herald DL, Arnold E, Clardy J (1982) Isolation and structure of bryostatin 1. J Am Chem Soc 104:6846–6848
Philip PA, Harris AL (1995) Potential for protein kinase C inhibitors in cancer therapy. In: Muggia FM (ed) Concepts, mechanisms and new targets for chemotherapy, 1st edn. Kluwer, Boston, pp 3–28
Philip PA, Rea D, Thavasu P, Carmichael J, Stuart NSA, Rockett H, Talbot DC, Ganesan T, Pettit GR, Balkwill F, Harris AL (1993) Phase I study of Bryostatin 1: assessment of interleukin 6 and tumor necrosis factor α induction in vivo. J Natl Cancer Inst 85:1812–1818
Pollack IF, Kawecki S, Lazo JS (1996) Blocking of glioma proliferation in vitro and in vivo and potentiating the effects of BCNU and cisplatin: UCN-01, a selective protein kinase C inhibitor. J Neurosurg 84:1024–1032
Prendiville J, Crowther D, Thatcher N, Woll PJ, Fox BW, McGown A, Testa N, Stern P, McDermott R, Potter M, Pettit GR (1993) A phase I study of intravenous bryostatin 1 in patients with advanced cancer. Br J Cancer 68:418–424
Roberts JD, Smith MR, Feldman EJ, Cragg L, Millenson MM, Roboz GJ, Honeycutt C, Thune R, Padavic-Shaller K, Carter WH, Ramakrishnan V, Murgo AJ, Grant S (2006) Phase I study of bryostatin 1 and fludarabine in patients with chronic lymphocytic leukemia and indolent (non-Hodgkin’s) lymphoma. Clin Cancer Res 12:5809–5816
Schuchter LM, Esa AH, May S, Laulis MK, Pettit GR, Hess AD (1991) Successful treatment of murine melanoma with bryostatin 1. Cancer Res 51:682–687
Szallasi Z, Du L, Levine R, Lewin NE, Nguyen PN, Williams MD, Pettit GR, Blumberg PM (1996) The bryostatins inhibit growth of B16/F10 melanoma cells in vitro through a protein kinase C-independent mechanism: dissociation of activities using 26-epi-bryostatin 1. Cancer Res 56:2105–2111
Thompson CH, Macaulay VM, O’Byrne KJ, Kemp GJ, Wilner SM, Talbot DC, Harris AL, Radda GK (1996) Modulation of bryostatin 1 muscle toxicity by nifedipine: effects on muscle metabolism and oxygen supply. Br J Cancer 73:1161–1165
Trenn G, Pettit GR, Takayama H, Hu-Li J, Sitkovsky MV (1988) Immunomodulating properties of a novel series of protein kinase C activators. The bryostatins. J Immunol 140:433–439
Varterasian M, Eilender D, Mohammad R, Chen B, Hulburd K, Rodriguez D, Pluda J, Valdivieso M, Al-Katib A (1996) Phase I trial of bryostatin 1 in relapsed lymphoma and CLL. Proc Am Soc Clin Oncol 15:481 (Abstr # 1526)
Weitman S, Langevin AM, Berkow RL, Thomas PJ, Hurwitz CA, Kraft AS, Dubowy AL, Smith DL, Bernstein M (1999) A phase I trial of bryostatin-1 in children with refractory solid tumors: a Pediatric Oncology Group study. Clin Cancer Res 5:2344–2348
WHO (1979) Handbook for reporting results of cancer treatment. Geneva (Switzerland): World Health Organization Offset Publication No. 48
Zhang R, Muller HJ, Kielassa K, Marks F, Gschwendt M (1994) Partial purification of a type eta protein kinase C from murine brain: separation from other protein kinase C isoenzymes and characterization. Biochem J 304:641–647
Zhao M, Rudek MA, He P, Smith BD, Baker SD (2005) Validation and implementation of a method for determination of bryostatin 1 in human plasma by using liquid chromatography/tandem mass spectrometry. Anal Biochem 33:143–148
Acknowledgment
The authors thank John Roboz for the GC/MS.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was supported by U01 CA76642, P30 CA 16087 and GCRC MO1 RR00096.
Rights and permissions
About this article
Cite this article
Pavlick, A.C., Wu, J., Roberts, J. et al. Phase I study of bryostatin 1, a protein kinase C modulator, preceding cisplatin in patients with refractory non-hematologic tumors. Cancer Chemother Pharmacol 64, 803–810 (2009). https://doi.org/10.1007/s00280-009-0931-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-009-0931-y