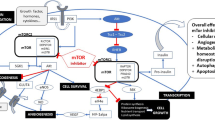
Abstract
Purpose
To detail tolerance of temsirolimus in a routine practice setting within a compassionate use program for patients with renal cell carcinoma.
Methods
We treated 32 patients with advanced renal cell carcinoma with temsirolimus within the German compassionate use program on an individual patient basis free of charge according to EU guidelines at our two institutions. Twenty-five milligrams of temsirolimus was applied weekly in an inpatient clinical setting. Adverse events were classified following National Cancer Institute Common Toxicity Criteria.
Results
No dose modification or therapy interruptions were necessary due to adverse events. Adverse events like asthenia/fatigue were observed in 43.8%, increased creatinine in 40.6%, mucositis in 31.3%, secondary diabetes in 28.1%, hypothyreosis in 12.5% and rash in 12.5%, hypercholesterolemia and hypertriglyceridemia in 9.3% of the patients.
Conclusion
Therapy with temsirolimus in advanced renal cell carcinoma is well tolerated. In a routine practice setting it results in a predictable adverse event profile that can be managed medically.
Similar content being viewed by others
Abbreviations
- TEMSR:
-
Temsirolimus
- CUP:
-
Compassionate use program
- AE:
-
Adverse event
- RCC:
-
Renal cell carcinoma
- EU:
-
European Union
- QoL:
-
Quality of life
- CTX:
-
Chemotherapy
- MSKCC:
-
Memorial Sloan-Kettering Cancer Center
- NCICTC:
-
National Cancer Institute Common Toxicity Criteria
- EMEA:
-
European Medicines Agency
- CHMP:
-
Committee for medicinal products for human use
References
Patard JJ, Pouessel D, Bensalah K, Culine S (2008) Targeted therapy in renal cell carcinoma. World J Urol 26(2):135–140
Trigo J, Bellmunt J (2008) Current strategies in the treatment of renal-cell cancer: targeted therapies. Med Clin 130(10):380–392
Pantuck AJ, Zeng G, Belldegrun AS, Figlin RA (2003) Pathobiology, prognosis, and targeted therapy for renal cell carcinoma: exploiting the hypoxia-induced pathway. Clin Cancer Res 9:4641–4652
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, Kovacevic Z, Lesovoy V, Schmidt-Wolf I, Barbarash O, Gokmen E, O’Toole T, Lustgarten S, Moore L, Motzer RJ (2007) Temsirolimus, Interferon Alfa, or both for advanced renal-cell carcinoma. N Engl J Med 356:2271–2281
Bhojani N, Jeldres C, Patard J, Perrotte P, Suardi N, Hutterer G, Patenaude F, Oudard S, Karakiewicz P (2008) Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol 53:917–930
Committee For Medicinal Products For Human Use (CHMP). Rules of Procedure EMEA/MB/87146/2007, EMEA/45110/2007. http://www.emea.europa.eu
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumours. J Nat Cancer Inst 92(3):205–216
Therasse P, Eisenhauer EA, Verweij J (2006) RECIST revisited: a review of validation studies on tumour assessment. Eur J Cancer 42:1031–1039
Erasmus JJ, Gladish GW, Broemeling L, Sabloff BS, Truong MT, Herbst RS, Munden RF (2003) Interobserver and intraobserver variability in measurement of non–small-cell carcinoma lung lesions: implications for assessment of tumor response. J Clin Oncol 21:2574–2582
Torisel [Package Insert]. Philadelphia, PA: Wyeth Pharmaceuticals, Inc. 2007
Cancer Therapy Evaluation Program (2006) Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS 2003, March 31, (http://ctep.cancer.gov)
Boni J, Leister C, Bender G, Fitzpatrick V, Twine N, Stover J, Dorner A, Immermann F, Burczynski ME (2005) Population pharmacokinetics of CCI-779: correlations to safety and pharmacogenomic responses in patients with advanced renal cancer. Clin Pharmacol Ther 77:76–89
Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, Leister C, Korth-Bradley J, Hanauske A, Armand JP (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 22:2336–2347
Buhaescu I, Izzedine H, Covic A (2006) Sirolimus–challenging current perspectives. Ther Drug Monit 28:577–584
Rangan GK (2006) Sirolimus-associated proteinuria and renal dysfunction. Drug Saf 29(12):1153–1161
Ubilla M, Mastrobuoni S, Cordero A, Ubilla M, Mastrobuoni S, Cordero A, Castaño S, Herreros J, Rabago G (2007) Impact on renal function of the use of sirolimus in cardiac transplantation. Transplant Proc 39:2401–2402
Duran I, Siu LL, Oza AM, Chung TB, Sturgeon J, Townsley CA, Pond GR, Seymour L, Niroumand M (2006) Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer 42:1875–1880
Pham TT, Pham CT, Danovitch GM, Ross DJ, Gritsch HA, Kendrick EA, Singer J, Shah T, Wilkinson AH (2004) Sirolimus-associated pulmonary toxicity. Transplant 77(8):1215–1220
Motzer RJ, Hutson TE, Tomczak P, Michaelson M, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Escudier B, Eisen T, Stadler W, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman Scott, Schwartz Brian, Shan Minghua, Simantov R, Bukowski RM (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Taha C, Liu Z, Jin J, Al-Hasani H, Sonenberg N, Klip A (1999) Opposite translational control of GLUT1 and GLUT4 glucose transporter mRNAs in response to insulin. Role of mammalian target of rapamycin, protein kinase b, and phosphatidylinositol 3-kinase in GLUT1 mRNA translation. J Biol Chem 274:33085–33091
Huffman TA, Mothe-Satney I, Lawrence JC Jr (2002) Insulin stimulated phosphorylation of lipin mediated by the mammalian target of rapamycin. Proc Natl Acad Sci USA 99:1047–1052
Acknowledgments
The authors thank Dr. C. Heuck for his excellent language support. All authors were investigators of the CUP. L. Bergmann and T. Otto were members of an advisory board of Wyeth Pharma, Germany. These positions had no influence on the outcome of the analysis.
Conflict of interest statement
There was no potential performance of this program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gerullis, H., Bergmann, L., Maute, L. et al. Experiences and practical conclusions concerning temsirolimus use and adverse event management in advanced renal cell carcinoma within a compassionate use program in Germany. Cancer Chemother Pharmacol 63, 1097–1102 (2009). https://doi.org/10.1007/s00280-008-0835-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-008-0835-2