Abstract
Purpose
Rubitecan is an oral camptothecin analogue that has shown activity against a broad spectrum of human tumor xenografts and has been tested in several diseases.
Patients and methods
In the present study, 19 patients with incurable, recurrent or metastatic head and neck cancer were treated with rubitecan at the initial dose of 1.5 mg/m2 × 5 days per week. An appropriate dose modification program was set up according to the observed toxicities.
Results
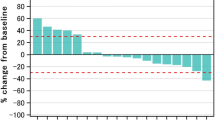
Thirteen out of the 19 treated patients were formally evaluable for tumor response. Ten patients had a disease progression and three patients had a stabilization of disease as their best response. The mean duration of stable disease was 141 days. Median survival was 16 weeks (range 2–22 weeks). Three patients died during the study or less than a month after their last dose of study medication. Hematologic toxicity was serious in this study since four patients discontinued their participation because of severe anemia. The drug was also associated with grade 1–4 neutropenia, and with 1–3 thrombocytopenia.
Conclusion
We conclude that rubitecan is not effective as a single-agent in recurrent or metastatic head and neck cancer with the doses and schedule used in this study.
Similar content being viewed by others
References
Amin A, Halabi S, Gelmann EP, Stadler W, Vogelzang N, Small E (2004) 9-Nitrocamptothecin as second line chemotherapy for menwith progressive, metastatic, hormone refractory prostate cancer: results of the CALGB 99901. Urol Oncol 22:398–403
Baka S, Ranson M, Lorigan P, Danson S, Linton K, Hoogendam I, Mettinger K, Thatcher N (2005) A phase II trial with RFS2000 (rubitecan) in patients with advanced non-small cell lung cancer. Eur J Cancer 41:1447–1550
Burris HA, Rivkin S, Reynolds R, Harris J, Wax A, Gerstein H, Mettinger KL, Staddon A (2005) Phase II trial oral rubitecan in previously treated pancreatic cancer. Oncologist 10:183–190
Caponigro F (2004) Rationale and clinical validation of epidermal growth factor receptor as a target in the treatment of head and neck cancer. Anticancer Drugs 15:311–320
Clark JW (2006) Rubitecan. Expert Opin Investig Drugs 15:71–79
de Jonge MJ, Droz JP, Paz-Ares L, van Oosterom AT, de Wit R, Chollet P, Baron B, Lacombe D, Mettinger K, Fumoleau P (2004) Phase II study on 9-nitrocamptothecin (RFS2000) in patients with advanced or metastatic urothelial tract tumors. Invest New Drugs 22:329–333
Ellerhorst JA, Bedikian AY, Smith TM, Papadopoulos NE, Plager C, Eton O (2002) Phase II trial of 9-nitrocamptothecin (RFS2000) for patients with metastatic cutaneous or uveal melanoma. Anticancer Drugs 13:169–172
Fracasso PM, Rader JS, Govindan R, Herzog TJ, Acquette MA, Denes A, Mutch DG, Picus J, Tan BR, Fears CL, Goodner SA, Sun SL (2002) Phase I study of rubitecan and gemcitabine in patients with advanced malignancies. Ann Oncol 13:1819–1825
Michaelson MD, Ryan DP, Fuchs CS, Supko JG, Garcia-Carbonero R, Paul Eder J, Clark JW (2003) A phase I study of 9-nitrocamptothecin given concurrently with capecitabine in patients with refractory, metastatic solid tumors. Cancer 97:148–154
Miller KD, Soule SE, Haney LG, Guiney P, Murry DJ, Lenaz L, Sun SL, Sledge GW (2004) A phase II study of 9-nitrocamptothecin in patients with previously treated metastatic breast cancer. Invest New Drugs 22:69–73
Patel H, Stoller R, Auber M, Potter D, Cai C, Zamboni W, Kiefer G, Matin K, Schmotzer A, Ramanathan RK (2006) Phase II of rubitecan, an oral camptothecin in patients with advanced colorectal who have failed previous 5-fluorouracil based chemotherapy. Invest New Drugs 24:359–363
Patel SR, Beach J, Padopoulos N, Burgess MA, Trent J, Jenkins J, Benjamin RS (2003) Results of a 2.arm phase II study of 9-nitrocamptothecin in patients with advanced soft-tissue sarcomas. Cancer 97:2848–2852
Punt CJ, de Jonge MJ, Monfardini S, Daugaard G, Fiedler W, Baron B, Lacombe D, Fumoleau P (2004) RFS2000 (9-nitrocamptothecin) in advanced small cell lung cancer, a phase II study of the EORTC new drug development group. Eur J Cancer 40:1332–1334
Raymond E, Campone M, Stupp R, Menten J, Chollet P, Lesimple T, Fety-Deporte R, Lacombe D, Paletti X, Fumoleau P (2002) Multicenter phase II and pharmacokinetic study of RFS2000 (9-nitrocamptothecin) administered orally 5 days a week in patients with glioblastoma multiforme. Eur J Cancer 38:1348–1350
Robert F, Soong SJ, Wheeler RH (1997) A phase II study of topotecan in patients with recurrent head and neck cancer. Identification of an active new agent. Am J Clin Oncol 20:298–302
Simon GR, Lush RM, Gump J, Tetteh L, Williams C, Cantor A, Antonia S, Garrett C, Rocha-Lima C, Fishman M, Sullivan DM, Munster PN (2006) Sequential oral 9-nitrocamptothecin and etoposide: a pharmacodynamic-and pharmacokinetic-based phase I trial. Mol Cancer Ther 5:2130–2137
Stehlin JS, Giovanella BC, Natelson EA, De Ipolyi PD, Coil D, Davis B, Davis B, Wolk D,Wallace P, Trojacek A (1999) A study of 9-nitrocamptothecin (RFS-2000) in patients with advanced pancreatic cancer. Int J Oncol 14:821–831
Tedesco KL, Berlin J, Rothenberg M, Choy H, Wyman K, Scott Pearson A, Daniel Beauchamp R, Merchant N, Lockhart AC, Shyr Y, Caillouette C, Chakravarthy B (2005) A phase I of concurrent 9-nitro-20(s)-camptothecin (9NC/orathecin) and radiation therapy in the treatment of locally advanced adenocarcinoma of the pancreas. Radiother Oncol 76:54–58
Zamboni WC, Jung LL, Egorin MJ, Potter DM, Friedland DM, Belani CP, Agarwala SS, Wong MM, Fakih M, Trump DL, Jin R, Strychor S, Vozniak M, Troetschel M, Ramanathan RK (2004) Phase I and pharmacologic study of the intermittently administered 9-nitrocamptothecin in patients with advanced solid tumors. Clin Cancer Res 10:5058–5064
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Caponigro, F., Cartenì, G., Droz, J.P. et al. Phase II study of rubitecan in recurrent or metastatic head and neck cancer. Cancer Chemother Pharmacol 62, 209–214 (2008). https://doi.org/10.1007/s00280-007-0592-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0592-7