Abstract
Purpose
Since a weekly administration of paclitaxel has demonstrated a sustained efficacy and more favorable toxicity profile than a 3-weekly administration for various solid tumors, the present study was conducted to evaluate the efficacy and safety of a combination regimen of weekly paclitaxel plus cisplatin in patients with advanced gastric cancer.
Patients and methods
Patients with previously untreated metastatic or recurrent, measurable gastric cancer received intravenous paclitaxel 100 mg/m2 plus cisplatin 35 mg/m2 on days 1 and 8 based on a 3-week cycle.
Results
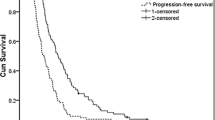
Fifty-two patients were enrolled in the current study. Two complete responses and 17 partial responses were confirmed, giving an overall response rate of 36.5%. At a median follow-up of 8.5 months, the median time to progression and median overall survival was 6.0 and 10.8 months, respectively. Grade 3 neutropenia occurred in ten patients, while no grade 4 neutropenia or febrile neutropenia was observed. The most common non-hematologic toxicity was nausea (grade 1/2, 56.9%). There were no treatment-related deaths.
Conclusion
A weekly paclitaxel and cisplatin combination was found to be well-tolerated and effective in patients with advanced gastric cancer. Accordingly, this regimen can be regarded as an important first-line treatment option for advanced gastric cancer.

Similar content being viewed by others
References
Pisani P, Parkin DM, Bray F, Ferlay J (1999) Estimates of the worldwide mortality from 25 cancers in 1990. Int J Cancer 83:18–29
Murad AM, Santiago FF, Petroianu A, Rocha PR, Rodrigues MA, Ransch M (1993) Modified therapy with 5-fluorouracil, doxorubicin, and methotrexate in advanced gastric cancer. Cancer 72:37–41
Pyrhonen S, Kuitunen T, Kouri M (1995) A randomized, phase III trial comparing fluorouracil, epidoxorubicin and methotrexate (FEMTX) with best supportive care in non-resectable gastric cancer. Br J Cancer 71:587–591
Glimelius B, Hoffmann K, Haglund U, Nyron O, Sjoden PO (1994) Initial or delayed chemotherapy with best supportive in advanced gastric cancer. Ann Oncol 5:189–190
Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC, Kang YK, Shin DB, Kim HT, Kim HJ, Kang WK, Suh CI, Bang YJ (1993) A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 71:3813–3818
Vanhoefer U, Wagner T, Lutz M, Van Cutsem E, Nordlinger B, Reuse S, Baron B, Wilke H, Wils J (2001) Randomized phase II study of weekly 24-h infusion of high dose 5-FU ± folinic acid (HD-FU ± FA) versus HD-FU/FA/biweekly cisplatin in advanced gastric cancer. EORTC-trial 40953. Eur J Cancer 37:27
Roth AD, Maibach R, Martinelli G, Fazio N, Aapro MS, Pagani O, Morant R, Borner MM, Hermann R, Honegger H, Cavalli F, Alberto P, Castighine M, Goldhirsch A (2000) Docetaxel (Taxotere)-cisplatin (TC): an effective drug combination in gastric carcinoma. Ann Oncol 11:301–306
Boku N, Ohtsu A, Shimada Y, Shirao K, Seki S, Saito H, Sakata Y, Hyodo I (1999) Phase II study of a combination of irinotecan and cisplatin against metastatic gastric cancer. J Clin Oncol 17:319–323
Schiff PB, Fant J, Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667
Jordan MA, Wendell K, Gardiner S, Derry WB, Copp H, Wilson L (1996) Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res 56:816–825
Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, Zhu J, Johnson DH (2002) Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 346:92–98
Ajani JA, Fairweather J, Dumas P, Patt YZ, Pazdur R, Mansfield PF (1998) Phase II study of Taxol in patients with advanced gastric carcinoma. Cancer J Sci Am 4:269–274
Bokemeyer C, Lampe CS, Clemens MR, Hartmann JT, Quietzsch D, Forkmann L, Kollmannsberger C, Kanz L (1997) A phase II trial of paclitaxel and weekly 24 h infusion of 5-fluorouracil/folinic acid in patients with advanced gastric cancer. Anticancer Drugs 8:396–399
Kollmannsberger C, Quietzsch D, Haag C, Lingenfelser T, Schroeder M, Hartmann JT, Baronius W, Hempd V, Uemens M, Kanz L, Bokemeyer C (2000) A phase II study of paclitaxel, weekly, 24-hour continuous infusion 5-fluorouracil, folinic acid and cisplatin in patients with advanced gastric cancer. Br J Cancer 83:458–462
Kim MK, Lee KH, Hyun MS, Do YR, Song HS, Lee WS, Park KU, Baek JH, Kim JG (2005) A multi-center, phase II clinical trial of Padexol (paclitaxel) and cisplatin for patients suffering with advanced gastric cancer. Cancer Res Treat 37:349–353
Georgiadis MS, Russell EK, Gazdar AF, Johnson BE (1997) Paclitaxel cytotoxicity against human lung cancer cell lines increases with prolonged exposure durations. Clin Cancer Res 3:449–454
Liebmann JE, Cook JA, Lipschultz C, Teague D, Fisher J, Mitchell JB (1993) Cytotoxic studies of paclitaxel (Taxol) in human tumor cell lines. Br J Cancer 68:1104–1109
Zhan Z, Scala S, Monks A, Hose C, Bates S, Fojo T (1997) Resistance to paclitaxel mediated by P-glycoprotein can be modulated by changes in the schedule of administration. Cancer Chemother Pharmacol 40:245–250
Akerley W, Herndon JE, Egorin MJ, Lyss AP, Kindler HL, Savarese DM, Sherman CA, Rosen DM, Hollis D, Ratain MJ, Green MR (2003) Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer 97:2480–2486
Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L (1998) Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol 16:3353–3361
Markman M, Hall J, Spitz D, Weiner S, Carson L, Van Le L, Baker M (2002) Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refractory ovarian cancer. J Clin Oncol 20:2365–2369
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan JS, Rubinstein L, Verweij J, Van Glabbeke M, Van Qosterom At, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Park SR, Oh DY, Kim DW, Kim TY, Heo DS, Bang YJ, Kim NK, Kang WK, Kim HT, Im SA, Suh JH, Kim HK (2004) A multi-center, late phase II clinical trial of genexol (paclitaxel) and cisplatin for patients with advanced gastric cancer. Oncol Rep 12:1059–1064
Kim YH, Shin SW, Kim BS, Kim JH, Kim JG, Mok YJ, Kim CS, Rhyu HS, Hyun JH, Kim JS (1999) Paclitaxel, 5-flourouracil, and cisplatin combination chemotherapy for the treatment of advanced gastric carcinoma. Cancer 85:295–301
Honecker F, Kollmannsberger C, Quietzsch D, Haag C, Schroeder M, Spott C, Hartmann JT, Baronius W, Hempel V, Kanz L, Bokemeyer C (2002) Phase II study of weekly paclitaxel plus 24-h continuous infusion 5-fluorouracil, folinic acid and 3-weekly cisplatin for the treatment of patients with advanced gastric cancer. Anticancer Drugs 13:497–503
Acknowledgment
This study was supported by a grant of the Korean Health 21 R&D Project, Ministry of Heath & Welfare, Republic of Korea (0412-CR01-0704-0001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, J.G., Sohn, S.K., Song, H.S. et al. Multicenter phase II study of weekly paclitaxel plus cisplatin combination chemotherapy in patients with advanced gastric cancer. Cancer Chemother Pharmacol 60, 863–869 (2007). https://doi.org/10.1007/s00280-007-0433-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0433-8