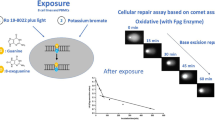
Abstract
Purpose
RH1 is a novel anticancer agent with potent DNA-cross linking activity. RH1 has the potential to be activated within tumors over expressing NQO1, giving maximal antitumour activity with reduced toxicity in normal tissues. RH1 has recently completed a Cancer Research UK sponsored phase I clinical trial at two different centers in the United Kingdom. The comet-X assay was a secondary endpoint in this trial and assay validation was necessary. We describe here this validation process. Whilst it is impossible to cover all variations/conditions of a pharmacodynamic assay, we have strived to evaluate and demonstrate that this assay conforms to the three R’s of validation, that is robustness, reliability and reproducibility.
Methods
K562 and peripheral blood mononuclear cells were treated with either radiation alone, or with a combination of radiation and drug. These samples were then embedded in low melting point agarose and subjected to a modified version of the alkaline single cell gel electrophoresis (Comet) assay, described here as the comet-X assay. Variations in the preparation, electrophoresis, storage and scoring of these samples was investigated. In addition radiation and drug dose response curves were constructed. Finally stability of QC standards was investigated over a 30-month period.
Results
We have demonstrated a linear radiation-dose response in cells up to 20 Gy and drug induced DNA cross-linking up to 50 nM. From the radiation dose response curves we were able to show that the relative inaccuracy measured against a global mean value was less than 25% and the relative (within day) imprecision was less than 30% over all doses. Between day runs produced an intra assay imprecision of 21.2%. Variables involved in the electrophoresis process showed the voltage across all slides in the tank ranged from 3.1 to −2.0 (mV) whilst the current ranged from 0.8–5.5 mA. QC standards were prepared from PBMCs of healthy donors and frozen at −80°C. The stability of these frozen QC standards was measured over a 30-month period. No significant deterioration in any of the control, irradiated or drug treated samples was observed.
Conclusions
The comet-X assay has been shown to be a robust, reliable and reproducible assay. It is ideally suited for the evaluation of the pharmacodynamic effects of DNA cross-linking agents undergoing early clinical trials. Furthermore, this assay may provide valuable data, in conjunction with pharmacokinetics, when measuring toxicity and efficacy as part of the RH1 phase I clinical trial.





Similar content being viewed by others
References
Ostling O, Johanson KJ (1984) Microelectrophoretic study of radiation-induced DNA damages in individual mammalian cells. Biochem Biophys Res Commun 123:291–298
Fairbairn DW, Olive PL, O’Neill KL (1995) The comet assay: a comprehensive review. Mutat Res 339:37–59
Olive PL, Banath JP (1993) Detection of DNA double-strand breaks through the cell cycle after exposure to X-rays, bleomycin, etoposide and 125IdUrd. Int J Radiat Biol 64:349–358
Moneef MA, Sherwood BT, Bowman KJ, Kockelbergh RC, Symonds RP, Steward WP, Mellon JK, Jones GD (2003) Measurements using the alkaline comet assay predict bladder cancer cell radiosensitivity. Br J Cancer 89:2271–2276
Woods JA, Young AJ, Gilmore IT, Morris ABilton RF (1997) Measurement of menadione-mediated DNA damage in human lymphocytes using the comet assay. Free Radic Res 26:113–124
Braybrooke JP, Houlbrook S, Crawley JE, Propper DJ, O’Byrne KJ, Stratford IJ, Harris AL, Shuker DE, Talbot DC (2000) Evaluation of the alkaline comet assay and urinary 3-methyladenine excretion for monitoring DNA damage in melanoma patients treated with dacarbazine and tamoxifen. Cancer Chemother Pharmacol 45:111–119
Tice RR, Strauss GH, Peters WP (1992) High-dose combination alkylating agents with autologous bone-marrow support in patients with breast cancer: preliminary assessment of DNA damage in individual peripheral blood lymphocytes using the single cell gel electrophoresis assay. Mutat Res 271:101–113
Vaghef H, Nygren P, Edling C, Bergh J, Hellman B (1997) Alkaline single-cell gel electrophoresis and human biomonitoring for genotoxicity: a pilot study on breast cancer patients undergoing chemotherapy including cyclophosphamide. Mutat Res 395:127–138
Hartley JM, Spanswick VJ, Gander M, Giacomini G, Whelan J, Souhami RL, Hartley JA (1999) Measurement of DNA cross-linking in patients on ifosfamide therapy using the single cell gel electrophoresis (comet) assay. Clin Cancer Res 5:507–512
Collins A, Dusinska M, Franklin M, Somorovska M, Petrovska H, Duthie S, Fillion L, Panayiotidis M, Raslova K, Vaughan N (1997) Comet assay in human biomonitoring studies: reliability, validation, and applications. Environ Mol Mutagen 30:139–146
Belpaeme K, Cooreman K, Kirsch-Volders M (1998) Development and validation of the in vivo alkaline comet assay for detecting genomic damage in marine flatfish. Mutat Res 415:167–184
De Boeck M, Touil N, De Visscher G, Vande PA, Kirsch-Volders M (2000) Validation and implementation of an internal standard in comet assay analysis. Mutat Res 469:181–197
Suggitt M, Fearnley J, Swaine DJ, Volpato M, Phillips RM, Bibby MC, Loadman PM, Anderson D (2003) Comet assay and flow cytometry analysis of DNA repair in normal and cancer cells treated with known mutagens with different mechanisms of action. Teratog Carcinog Mutagen Suppl 2:13–29
Ward TH, Butler J, Shahbakhti H, Richards JT (1997) Comet assay studies on the activation of two diaziridinylbenzoquinones in K562 cells. Biochem Pharmacol 53:1115–1121
Friedmann B, Caplin M, Hartley JA, Hochhauser D (2004) Modulation of DNA repair in vitro after treatment with chemotherapeutic agents by the epidermal growth factor receptor inhibitor gefitinib (ZD1839). Clin Cancer Res 10:6476–6486
Dehn DL, Winski SL, Ross D (2004) Development of a new isogenic cell-xenograft system for evaluation of NAD(P)H:quinone oxidoreductase-directed antitumor quinones: evaluation of the activity of RH1. Clin Cancer Res 10:3147–3155
Winski SL, Hargreaves RH, Butler J, Ross D (1998) A new screening system for NAD(P)H:quinone oxidoreductase (NQO1)-directed antitumor quinones: identification of a new aziridinylbenzoquinone, RH1, as a NQO1-directed antitumor agent. Clin Cancer Res 4:3083–3088
Loadman PM, Phillips RM, Lim LE, Bibby MC (2000) Pharmacological properties of a new aziridinylbenzoquinone, RH1 (2,5-diaziridinyl-3-(hydroxymethyl)-6-methyl-1,4-benzoquinone), in mice. Biochem Pharmacol 59:831–837
Cummings J, Ritchie A, Butler J, Ward TH, Langdon S (2003) Activity profile of the novel aziridinylbenzoquinones MeDZQ and RH1 in human tumour xenografts. Anticancer Res 23:3979–3983
Kim JY, Patterson AV, Stratford IJ, Hendry JH (2004) The importance of DT-diaphorase and hypoxia in the cytotoxicity of RH1 in human breast and non-small cell lung cancer cell lines. Anticancer Drugs 15:71–77
Ward TH, Danson S, McGown AT, Ranson M, Coe NA, Jayson GC, Cummings J, Hargreaves RH, Butler J (2005) Preclinical evaluation of the pharmacodynamic properties of 2,5-diaziridinyl-3-hydroxymethyl-6-methyl-1,4-benzoquinone. Clin Cancer Res 11:2695–2701
Tudor G, Alley M, Nelson CM, Huang R, Covell DG, Gutierrez P, Sausville EA (2005) Cytotoxicity of RH1: NAD(P)H:quinone acceptor oxidoreductase (NQO1)-independent oxidative stress and apoptosis induction. Anticancer Drugs 16:381–391
Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF (2000) Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen 35:206–221
Moller P (2006) The alkaline comet assay: towards validation in biomonitoring of DNA damaging exposures. Basic Clin Pharmacol Toxicol 98:336–345
Rosengren NFaB (2001) Directive 2001/20/EC of the European Parliment and of the Council of 4 April 2001 on the approximation of the laws, regulations and administrative provisions of the Member States relating to the implementation of good clinical practice in the conduct of clinical trials on medicinal products for human use. Official J Eur Communities L121:34–44
Rothenberg ML, Carbone DP, Johnson DH (2003) Improving the evaluation of new cancer treatments: challenges and opportunities. Nat Rev Cancer 3:303–309
Woude GF, Kelloff GJ, Ruddon RW, Koo HM, Sigman CC, Barrett JC, Day RW, Dicker AP, Kerbel RS, Parkinson DR, Slichenmyer WJ (2004) Reanalysis of cancer drugs: old drugs, new tricks. Clin Cancer Res 10:3897–3907
Shah VP, Midha KK, Findlay JW, Hill HM, Hulse JD, McGilveray IJ, McKay G, Miller KJ, Patnaik RN, Powell ML, Tonelli A, Viswanathan CT, Yacobi A (2000) Bioanalytical method validation–a revisit with a decade of progress. Pharm Res 17:1551–1557
Miller KJ, Bowsher RR, Celniker A, Gibbons J, Gupta S, Lee JW, Swanson SJ, Smith WC, Weiner RS (2001) Workshop on bioanalytical methods validation for macromolecules: summary report. Pharm Res 18:1373–1383
Collins AR, Dusinska M, Gedik CM, Stetina R (1996) Oxidative damage to DNA: do we have a reliable biomarker? Environ Health Perspect 104(suppl 3):465–469
Olive PL, Banath JP, Durand RE (1990) Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the “comet” assay. Radiat Res 122:86–94
Oppitz U, Schulte S, Stopper H, Baier K, Muller M, Wulf J, Schakowski R, Flentje M (2002) In vitro radiosensitivity measured in lymphocytes and fibroblasts by colony formation and comet assay: comparison with clinical acute reactions to radiotherapy in breast cancer patients. Int J Radiat Biol 78:611–616
Acknowledgments
This work was supported and funded by Cancer Research UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Danson, S., Ranson, M., Denneny, O. et al. Validation of the comet-X assay as a pharmacodynamic assay for measuring DNA cross-linking produced by the novel anticancer agent RH1 during a phase I clinical trial. Cancer Chemother Pharmacol 60, 851–861 (2007). https://doi.org/10.1007/s00280-007-0432-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0432-9