Abstract
Purpose
Etoposide is a widely used cytotoxic drug that is commercially available in both intravenous and oral formulations. High interpatient pharmacokinetic variability has been associated with oral etoposide administration. Various strategies used in the past to reduce such variability have not been successful. Hence, this study was designed to evaluate if pharmacokinetic modulation of oral etoposide with ketoconazole could lead to a favorable alteration of etoposide pharmacokinetics, and to assess the feasibility and safety of this approach.
Methods
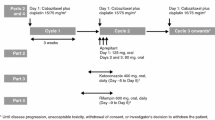
Thirty-two patients were treated with ketoconazole 200 mg daily with an escalating dose of oral etoposide starting at a dose of 50 mg every other day. Pharmacokinetic samples were obtained during the first treatment cycle after the administration of an oral etoposide and ketoconazole dose. Additional baseline pharmacokinetic studies of etoposide alone were performed 4 days prior to the first treatment cycle.
Results
Dose limiting toxicities were neutropenia and fatigue. Ketoconazole increased the area under the plasma concentration–time curve (AUC) of oral etoposide by a median of 20% (p < 0.005). Ketoconazole did not reduce the interpatient variability in etoposide pharmacokinetics. Pretreatment bilirubin levels correlated with etoposide clearance (Spearman’s r = −0.48, p = 0.008). The maximum tolerated dose was etoposide administered at 50 mg daily and ketoconazole 200 mg qd for 3 of 5 weeks.
Conclusions
Ketoconazole reduces the apparent clearance of oral etoposide, does not alter its toxicity profile and does not reduce interpatient pharmacokinetic variability. Other methods to reduce the pharmacokinetic variability of oral etoposide are needed.


Similar content being viewed by others
References
Ahmed FY, Johnston SJ, Cassidy J, O’Kelly T, Binnie N, Murray GI, van Gennip AH, Abeling NG, Knight S, McLeod HL (1999) Eniluracil treatment completely inactivates dihydropyrimidine dehydrogenase in colorectal tumors. J Clin Oncol 17:2439–2445
Aita P, Robieux I, Sorio R, Tumolo S, Corona G, Cannizzaro R, Colussi AM, Boiocchi M, Toffoli G (1999) Pharmacokinetics of oral etoposide in patients with hepatocellular carcinoma. Cancer Chemother Pharmacol 43:287–294
Ando Y, Saka H, Asai G, Sugiura S, Shimokata K, Kamataki T (1998) UGT1A1 genotypes and glucuronidation of SN-38, the active metabolite of irinotecan. Ann Oncol 9:845–847
Arbuck SG, Douglass HO, Crom WR, Goodwin P, Silk Y, Cooper C, Evans WE (1986) Etoposide pharmacokinetics in patients with normal and abnormal organ function. J Clin Oncol 4:1690–1695
Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP, Chowdhury NR (1995) The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333:1171–1175
Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL (1994) Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem 269:17960–17964
Buss N, Snell P, Bock J, Hsu A, Jorga K (2001) Saquinavir and ritonavir pharmacokinetics following combined ritonavir and saquinavir (soft gelatin capsules) administration. Br J Clin Pharmacol 52:255–264
Chen GL, Yang L, Rowe TC, Halligan BD, Tewey KM, Liu LF (1984) Nonintercalative antitumor drugs interfere with the breakage-reunion reaction of mammalian DNA topoisomerase II. J Biol Chem 259:13560–13566
D’Incalci M, Rossi C, Zucchetti M, Urso R, Cavalli F, Mangioni C, Willems Y, Sessa C (1986) Pharmacokinetics of etoposide in patients with abnormal renal and hepatic function. Cancer Res 46:2566–2571
Desai AA, Innocenti F, Janisch L, DeMario M, Shepard D, Ramirez J, Fleming G, Ratain MJ (2004) A phase I trial of pharmacokinetic modulation of carboxyamidotriazole (CAI) with ketoconazole in patients with advanced cancer. Cancer Chemother Pharmacol 54:377–384
el-Yazigi A, Ezzat A, Berry J, Raines DA, Yusuf A, al-Rawithi S, Legayada ES (2000) Optimization of oral etoposide dosage in elderly patients with non-Hodgkin’s lymphoma using the fraction of dose absorbed measured for each patient. J Clin Pharmacol 40:153–160
Engels FK, Ten Tije AJ, Baker SD, Lee CK, Loos WJ, Vulto AG, Verweij J, Sparreboom A (2004) Effect of cytochrome P450 3A4 inhibition on the pharmacokinetics of docetaxel. Clin Pharmacol Ther 75:448–454
Fleming RA, Evans WE, Arbuck SG, Stewart CF (1992) Factors affecting in vitro protein binding of etoposide in humans. J Pharm Sci 81:259–264
Gibbs MA, Kunze KL, Howald WN, Thummel KE (1999) Effect of inhibitor depletion on inhibitory potency: tight binding inhibition of CYP3A by clotrimazole. Drug Metab Dispos 27:596–599
Hande K, Anthony L, Hamilton R, Bennett R, Sweetman B, Branch R (1988) Identification of etoposide glucuronide as a major metabolite of etoposide in the rat and rabbit. Cancer Res 48:1829–1834
Hande K, Messenger M, Wagner J, Krozely M, Kaul S (1999) Inter- and intrapatient variability in etoposide kinetics with oral and intravenous drug administration. Clin Cancer Res 5:2742–2747
Hande KR, Krozely MG, Greco FA, Hainsworth JD, Johnson DH (1993) Bioavailability of low-dose oral etoposide. J Clin Oncol 11:374–377
Hande KR, Wolff SN, Greco FA, Hainsworth JD, Reed G, Johnson DH (1990) Etoposide kinetics in patients with obstructive jaundice. J Clin Oncol 8:1101–1107
Harvey VJ, Slevin ML, Joel SP, Johnston A, Wrigley PF (1986) The effect of dose on the bioavailability of oral etoposide. Cancer Chemother Pharmacol 16:178–181
Hustert E, Zibat A, Presecan-Siedel E, Eiselt R, Mueller R, Fuss C, Brehm I, Brinkmann U, Eichelbaum M, Wojnowski L, Burk O (2001) Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab Dispos 29:1454–1459
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE, Ratain MJ (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382–1388
Ito K, Brown HS, Houston JB (2004) Database analyses for the prediction of in vivo drug-drug interactions from in vitro data. Br J Clin Pharmacol 57:473–486
Joel SP, Clark PI, Heap L, Webster L, Robbins S, Craft H, Slevin ML (1995) Pharmacological attempts to improve the bioavailability of oral etoposide. Cancer Chemother Pharmacol 37:125–133
Kaul S, Srinivas NR, Igwemezie LN, Barbhaiya RH (1996) A pharmacodynamic evaluation of hematologic toxicity observed with etoposide phosphate in the treatment of cancer patients. Semin Oncol 23:15–22
Kuehl P, Zhang J, Lin Y, Lamba J, Assem M, Schuetz J, Watkins PB, Daly A, Wrighton SA, Hall SD, Maurel P, Relling M, Brimer C, Yasuda K, Venkataramanan R, Strom S, Thummel K, Boguski MS, Schuetz E (2001) Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet 27:383–391
Lum BL, Kaubisch S, Yahanda AM, Adler KM, Jew L, Ehsan MN, Brophy NA, Halsey J, Gosland MP, Sikic BI (1992) Alteration of etoposide pharmacokinetics and pharmacodynamics by cyclosporine in a phase I trial to modulate multidrug resistance. J Clin Oncol 10:1635–1642
Malingre MM, Richel DJ, Beijnen JH, Rosing H, Koopman FJ, Ten Bokkel Huinink WW, Schot ME, Schellens JH (2001) Coadministration of cyclosporine strongly enhances the oral bioavailability of docetaxel. J Clin Oncol 19:1160–1166
Maurice M, Pichard L, Daujat M, Fabre I, Joyeux H, Domergue J, Maurel P (1992) Effects of imidazole derivatives on cytochromes P450 from human hepatocytes in primary culture. Faseb J 6:752–758
Meerum Terwogt JM, Malingre MM, Beijnen JH, ten Bokkel Huinink WW, Rosing H, Koopman FJ, van Tellingen O, Swart M, Schellens JH (1999) Coadministration of oral cyclosporin A enables oral therapy with paclitaxel. Clin Cancer Res 5:3379–3384
Millward MJ, Newell DR, Yuen K, Matthews JP, Balmanno K, Charlton CJ, Gumbrell L, Lind MJ, Chapman F, Proctor M, et al (1995) Pharmacokinetics and pharmacodynamics of prolonged oral etoposide in women with metastatic breast cancer. Cancer Chemother Pharmacol 37:161–167
Monaghan G, Ryan M, Seddon R, Hume R, Burchell B (1996) Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 347:578–581
O’Dwyer PJ, Leyland-Jones B, Alonso MT, Marsoni S, Wittes RE (1985) Etoposide (VP-16–213). Current status of an active anticancer drug. N Engl J Med 312:692–700
Paredes J, Kahn JO, Tong WP, Feldstein ML, Lin S, Bennett JM, Metroka CE, Ratner L, Krown SE (1995) Weekly oral etoposide in patients with Kaposi’s sarcoma associated with human immunodeficiency virus infection: a phase I multicenter trial of the AIDS Clinical Trials Group. J Acquir Immune Defic Syndr Hum Retrovirol 9:138–144
Patki KC, Von Moltke LL, Greenblatt DJ (2003) In vitro metabolism of midazolam, triazolam, nifedipine, and testosterone by human liver microsomes and recombinant cytochromes p450: role of cyp3a4 and cyp3a5. Drug Metab Dispos 31:938–944
Perdaems N, Bachaud JM, Rouzaud P, Murris-Espin M, Hermant C, Mihura J, Lochon I, Houin G, Canal P, Chatelut E (1998) Relation between unbound plasma concentrations and toxicity in a prolonged oral etoposide schedule. Eur J Clin Pharmacol 54:677–683
Relling MV, Evans R, Dass C, Desiderio DM, Nemec J (1992) Human cytochrome P450 metabolism of teniposide and etoposide. J Pharmacol Exp Ther 261:491–496
Relling MV, Nemec J, Schuetz EG, Schuetz JD, Gonzalez FJ, Korzekwa KR (1994) O-demethylation of epipodophyllotoxins is catalyzed by human cytochrome P450 3A4. Mol Pharmacol 45:352–358
Satoh T, Tomikawa Y, Takanashi K, Itoh S, Yoshizawa I (2004) Studies on the interactions between drugs and estrogen. III. Inhibitory effects of 29 drugs reported to induce gynecomastia on the glucuronidation of estradiol. Biol Pharm Bull 27:1844–1849
Sessa C, Zucchetti M, Torri V, Pagani O, D’Incalci M, Gentili D, Martinelli G, deJong J, Alerci M, Cavalli F (1993) Chronic oral etoposide in small-cell lung cancer: clinical and pharmacokinetic results. Ann Oncol 4:553–558
Sinkule JA, Hutson P, Hayes FA, Etcubanas E, Evans W (1984) Pharmacokinetics of etoposide (VP16) in children and adolescents with refractory solid tumors. Cancer Res 44:3109–3113
Slevin ML, Clark PI, Joel SP, Malik S, Osborne RJ, Gregory WM, Lowe DG, Reznek RH, Wrigley PF (1989) A randomized trial to evaluate the effect of schedule on the activity of etoposide in small-cell lung cancer. J Clin Oncol 7:1333–1340
Slevin ML, Joel SP (1993) Prolonged oral etoposide in small cell lung cancer. Ann Oncol 4:529–532
Smyth RD, Pfeffer M, Scalzo A, Comis RL (1985) Bioavailability and pharmacokinetics of etoposide (VP-16). Semin Oncol 12:48–51
Stewart CF, Arbuck SG, Fleming RA, Evans WE (1990) Changes in the clearance of total and unbound etoposide in patients with liver dysfunction. J Clin Oncol 8:1874–1879
Stewart CF, Fleming RA, Arbuck SG, Evans WE (1990) Prospective evaluation of a model for predicting etoposide plasma protein binding in cancer patients. Cancer Res 50:6854–6856
Toffoli G, Corona G, Basso B, Boiocchi M (2004) Pharmacokinetic optimisation of treatment with oral etoposide. Clin Pharmacokinet 43:441–466
Toffoli G, Corona G, Sorio R, Robieux I, Basso B, Colussi AM, Boiocchi M (2001) Population pharmacokinetics and pharmacodynamics of oral etoposide. Br J Clin Pharmacol 52:511–519
van der Gaast A, Vlastuin M, Kok TC, Splinter TA (1992) What is the optimal dose and duration of treatment with etoposide? II. Comparative pharmacokinetic study of three schedules: 1 × 100 mg, 2 × 50 mg, and 4 × 25 mg of oral etoposide daily for 21 days. Semin Oncol 19:8–12
Van Veldhuizen PJ, Reed G, Aggarwal A, Baranda J, Zulfiqar M, Williamson S (2003) Docetaxel and ketoconazole in advanced hormone-refractory prostate carcinoma: a phase I and pharmacokinetic study. Cancer 98:1855–1862
Watanabe Y, Nakajima M, Ohashi N, Kume T, Yokoi T (2003) Glucuronidation of etoposide in human liver microsomes is specifically catalyzed by UDP-glucuronosyltransferase 1A1. Drug Metab Dispos 31:589–595
Yahanda AM, Alder KM, Fisher GA, Brophy NA, Halsey J, Hardy RI, Gosland MP, Lum BL, Sikic BI (1992) Phase I trial of etoposide with cyclosporine as a modulator of multidrug resistance. J Clin Oncol 10:1624–1634
Yong WP, Ramirez J, Innocenti F, Ratain MJ (2005) Effects of ketoconazole on glucuronidation by UDP-glucuronosyltransferase enzymes. Clin Cancer Res 11:6699–6704
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yong, W.P., Desai, A.A., Innocenti, F. et al. Pharmacokinetic modulation of oral etoposide by ketoconazole in patients with advanced cancer. Cancer Chemother Pharmacol 60, 811–819 (2007). https://doi.org/10.1007/s00280-007-0428-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0428-5