Abstract
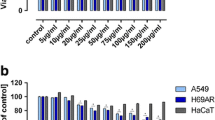
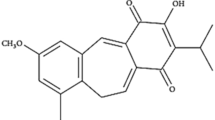
To develop a new taxon of anti-cancer agent with lower side effect, this study described a tumor selective cytotoxicity of glucosylceramide extracted from malt feed of beer brewing waste. Interpretation of 13C- and 1H-NMR spectra identified the chemical structure of major component of glucosylceramide as 1-O-β-d-glucopyranosyl-2(2′-hydroxyeicosanoylamino)-4,11-octadecadiene-1,3-diol. Selective cytotoxicity was studied with three pairs of normal and cancer cells: liver, skin and lung. The glucosylceramide selectively lowered the relative viability of cancer cells. Of the pairs, the selectivity was most pronounced with the liver cells, and, for this reason, further experiment was conducted with this pair of normal (CS-HC) and cancer cells (HepG2) to get more insight into the selective toxicity. The glucosylceramide significantly increased the cell population at G2/M phase in HepG2 cells, and also increased the numbers of apoptotic (sub-G0/G1) cells, but to much lesser extent compared with the increase in G2/M phase. Treatment of HepG2 cells with this agent selectively disrupted the mitochondrial membrane integrity without activation of caspase pathway to induce apoptosis. These findings suggested that the glucosylceramide specifically suppressed the growth of cancer cells by inhibiting cell renewal capacity rather than induction of apoptosis. The underlying mechanism for the selectivity remains to be answered in the forthcoming study.





Similar content being viewed by others
References
Sullards MC, Lynch DV, Merrill AH Jr, Adams J (2000) Structure determination of soybean and wheat glucosylceramides by tandem mass spectrometry. J Mass Spectrom 35:347–353
Schmelz EM, Sullards MC, Dillehay DL, Merrill AH Jr (1999) Colonic cell proliferation and aberrant crypt foci formation are inhibited by dairy glucosphingolipids in 1,2-demethylhydrazine-treated CF1 mice. J Nutr 130:522–527
Symolon H, Schmelz EM, Dillehay DL, Merrill AH Jr (2004) Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J Nutr 134:1157–1161
Inamine M, Suzui M, Morioka T, Kinjo T, Kaneshiro T, Sugishita T, Okada T, Yoshimi N (2005) Inhibitory effect of dietary monoglucosylceramide 1-O-beta-glucosyl-N-2′-hydroxyarachidoyl-4,8-sphingadienine on two different categories of colon preneoplastic lesions induced by 1,2-dimethylhydrazine in F344 rats. Cancer Sci 96:876–881
Chen JH, Cui GY, Liu JY, Tan RX (2003) Pinelloside, an antimicrobial cerebroside from Pinellia ternata. Phytochemistry 64:903–906
Cateni F, Zilic J, Falsone G, Scialino G, Banfi E (2003) New cerebrosides from Euphorbia peplis L.: antimicrobial activity evaluation. Bioorg Med Chem Lett 13:4345–4350
Bieberich E (2004) Integration of glycosphingolipid metabolism and cell-fate decisions in cancer and stem cells: review and hypothesis. Glycoconj J 21:315–327
van Blitterswijk WJ, van der Luit AH, Veldman RJ, Verheij M, Borst J (2003) Ceramide: second messenger or modulator of membrane structure and dynamics? Biochem J 369:199–211
Naka T, Fujiwara N, Yano I, Maeda S, Doe M, Minamino M, Ikeda N, Kato Y, Watabe K, Kumazawa Y, Tomiyasu I, Kobayashi K (2003) Structural analysis of sphingophospholipids derived from Sphingobacterium spiritivorum, the type species of genus Sphingobacterium. Biochim Biophys Acta 1635:83–92
Cateni F, Zilic J, Falsone G, Hollan F, Frausin F, Scarcia V (2003) Preliminary biological assay on cerebroside mixture from Euphorbia nicaeensis All. Isolation and structure determination of five glucocerebrosides. Farmaco 58:809–817
Rupcic J, Maric V (2004) Cerebrosides of Candida lipolytica yeast. Appl Microbiol Biotechnol 64:416–420
Takakuwa N, Saito K, Ohnishi M, Oda Y (2005) Determination of glucosylceramide contents in crop tissues and by-products from their processing. Bioresour Technol 96:1089–1092
Wongtangtintharn S, Oku H, Iwasaki H, Inafuku M, Toda T, Yanagita T (2005) Incorporation of branched-chain fatty acid into cellular lipids and caspase-independent apoptosis in human breast cancer cell line, SKBR-3. Lipids Health Dis 4:29
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Christie WW (1985) Rapid separation and quantification of lipid classes by high performance liquid chromatography and mass (light-scattering) detection. J Lipid Res 26:507–512
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bras M, Queenan B, Susin SA (2005) Programmed cell death via mitochondria: different modes of dying. Biochemistry (Mosc) 70:231–239
Kobayashi E, Motoki K, Yamaguchi Y, Uchida T, Fukushima H, Koezuka Y (1996) Enhancing effects of alpha-, beta-monoglycosylceramides on natural killer cell activity. Bioorg Med Chem 4:615–619
Ishihara S, Nieda M, Kitayama J, Osada T, Yabe T, Kikuchi A, Koezuka Y, Porcelli SA, Tadokoro K, Nagawa H, Juji T (2000) Alpha-glycosylceramides enhance the antitumor cytotoxicity of hepatic lymphocytes obtained from cancer patients by activating CD3-CD56+ NK cells in vitro. J Immunol 165:1659–1664
Zhou D, Mattner J, Cantu C III, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A (2004) Lysosomal glycosphingolipid recognition by NKT cells. Science 306:1786–1789
Maru M, Haraguchi M, Higashi H, Kato S, Kurimura T, Naiki M, Wakamiya N (1993) Anti-tumor activity of ceramides and glycosphingolipids in a murine tumor system. Int J Cancer 53:645–650
Schmelz EM, Merrill AH Jr (1998) Ceramides and ceramide metabolites in cell regulation: evidence for dietary sphingolipids as inhibitors of colon carcinogenesis. Nutrition 14:717–719
Sugiki H, Hozumi Y, Maeshima H, Katagata Y, Mitsuhashi Y, Kondo S (2000) C2-ceramide induces apoptosis in a human squamous cell carcinoma cell line. Br J Dermatol 143:1154–1163
Ogretmen B, Schady D, Usta J, Wood R, Kraveka JM, Luberto C, Birbes H, Hannun YA, Obeid LM (2001) Role of ceramide in mediating the inhibition of telomerase activity in A549 human lung adenocarcinoma cells. J Biol Chem 276:24901–24910
Morales A, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC (2004) Glycosphingolipids and mitochondria: role in apoptosis and disease. Glycoconj J 20:579–588
Mochizuki T, Asai A, Saito N, Tanaka S, Katagiri H, Asano T, Nakane M, Tamura A, Kuchino Y, Kitanaka C, Kirino T (2002) Akt protein kinase inhibits non-apoptotic programmed cell death ilduced by ceramide. J Biol Chem 277:2790–2797
von Haefen C, Wieder T, Gillissen B, Starck L, Graupner V, Dorken B, Daniel PT (2002) Ceramide induces mitochondrial activation and apoptosis via a Bax-dependent pathway in human carcinoma cells. Oncogene 21:4009–4019
Kuroki J, Hirokawa M, Kitabayashi A, Lee M, Horiuchi T, Kawabata Y, Miura AB (1996) Cell-permeable ceramide inhibits the growth of B lymphoma Raji cells lacking TNF-alpha-receptors by inducing G0/G1 arrest but not apoptosis: a new model for dissecting cell-cycle arrest and apoptosis. Leukemia 10:1950–1958
Connor CE, Burrows J, Hearps AC, Woods GM, Lowenthal RM, Ragg SJ (2001) Cell cycle arrest of hematopoietic cell lines after treatment with ceramide is commonly associated with retinoblastoma activation. Cytometry 43:164–169
Takai N, Ueda T, Kawano Y, Nishida M, Nasu K, Narahara H (2005) C2-ceramide exhibits antiproliferative activity and potently induces apoptosis in endometrial carcinoma. Oncol Rep 14:1287–1291
Ahn EH, Schroeder JJ (2002) Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp Biol Med (Maywood) 227:345–353
Acknowledgment
The authors thank Ms. Shiho Tomori for her excellent technical assistance, and our thanks also go to Orion Beer Co. and DNA bank Co. for the preparation of crude ceramide from malt feed.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oku, H., Wongtangtintharn, S., Iwasaki, H. et al. Tumor specific cytotoxicity of glucosylceramide. Cancer Chemother Pharmacol 60, 767–775 (2007). https://doi.org/10.1007/s00280-007-0422-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-007-0422-y