Abstract
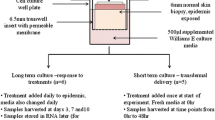
There are potential advantages to detecting pharmacodynamic (PD) and pharmacokinetic (PK) endpoints in a tissue-based compartment such as the skin during the development of molecularly targeted drugs. We explored regional differences between inner arm, inner thigh, lower back and buttocks in 12 healthy male Caucasian volunteers in the tolerability of skin biopsy procedures; the Ki67 proliferation index; the frequency of detecting hair follicles and sweat glands; and the percentage of melanocytes. We also explored the amounts of tissue and protein obtained, and two separate methods of splitting biopsies for processing in mutually exclusive media. Biopsies from all body sites were well tolerated. The subjective ranking order was inner arm > buttocks = back > thigh. There were no statistically significant differences in the Ki67 labelling index (P>0.05). The frequency of detecting sweat glands was the same in all body sites, but the frequency of detecting hair follicles was higher in back and buttock, compared to arm and thigh. The percentage of melanocytes was significantly lower in the buttocks compared to the back and thigh (P<0.05), but not compared to the arm (P=0.07). A 4-mm punch biopsy yielded a mean of 16.8 mg of tissue (range: 9–28 mg) and 160 μg of protein (range: 80–270 μg). In vivo sample splitting, by following a 2-mm punch with a 4-mm overpunch, had a shorter time from devascularisation to immersion into processing medium than ex vivo dissection of a 4-mm sample, which may be of importance to the assessment of labile endpoints. We conclude that multiple punch biopsies of the skin are feasible, with the buttocks representing the studied body site with the optimal balance between tolerability, hair follicle density and melanocyte density for obtaining tissue in which to assess PD and PK endpoints during drug development studies.

Similar content being viewed by others
Abbreviations
- IHC:
-
Immunohistochemistry
- CPU:
-
Clinical Pharmacology Unit
- EGFR:
-
Epidermal growth factor receptor
- PK:
-
Pharmacokinetic
- PD:
-
Pharmacodynamic
- GCP:
-
Good clinical practice
- ICH:
-
International conference on harmonisation
- mg:
-
Milligram
- μg:
-
Microgram
- rpm:
-
Revolutions per minute
- H & E:
-
Haematoxylin and eosin
- HPLC:
-
High pressure liquid chromatography
- LCMS:
-
Liquid chromatography mass spectrometry
- ELISA:
-
Enzyme-linked immunosorbent assay
- ANOVA:
-
Analysis of variance
- mm:
-
Millimetre
- p:
-
Probability
- mL:
-
Millilitre
- g:
-
Gram
- μL:
-
Microlitre
- NaCl:
-
Sodium chloride
- mM:
-
Millimolar
- CL:
-
Confidence limit
- Glsmean:
-
Geometric least squares mean
- n/a:
-
Non-applicable
References
Albanell J, Rojo F, Averbuch S, Feyereislova A, Mascaro JM, Herbst R, LoRusso P, Rischin D, Sauleda S, Gee J, Nicholson RI, Baselga J (2002) Pharmacodynamic studies of the epidermal growth factor receptor inhibitor ZD1839 in skin from cancer patients: histopathologic and molecular consequences of receptor inhibition. J Clin Oncol 20:110–124
Alster TS, Tanzi EL (2003) Hypertrophic scars and keloids: etiology and management. Am J Clin Dermatol 4:235–243
Baselga J (2003) Skin as a surrogate tissue for pharmacodynamic end points: is it deep enough? Clin Cancer Res 9:2389–2390
Del Bino S, Vioux C, Rossio-Pasquier P, Jomard A, Demarchez M, Asselineau D, Bernerd F (2004) Ultraviolet B induces hyperproliferation and modification of epidermal differentiation in normal human skin grafted on to nude mice. Br J Dermatol 150:658–667
Hidalgo M, Messersmith W (2004) Pharmacodynamic studies in drug development. In: Michael C, Perry MC (ed) American society of clinical oncology educational book, Alexandria, VA, pp 160–163. ISSN 1548–8748
Larsson BS (1993) Interaction between chemicals and melanin. Pigment Cell Res 6:127–133
Malik SN, Siu LL, Rowinsky EK, deGraffenreid L, Hammond LA, Rizzo J, Bacus S, Brattain MG, Kreisberg JI, Hidalgo M (2003) Pharmacodynamic evaluation of the epidermal growth factor receptor inhibitor OSI-774 in human epidermis of cancer patients. Clin Cancer Res 9:2478–2486
Mandell JW (2003) Phosphorylation state-specific antibodies. Am J Pathol 163:1687–1698
McKillop D, Raab G, Eidtmann H, Furnival A, Riva A, Forbes J, Mackey J, Spence MP, Koehler M, Slamon D (2004) Intratumoral and plasma concentrations of gefitinib in breast cancer patients: preliminary results from a presurgical investigatory study (BCIRG 103). In: Proceedings of the American Society of Clinical Oncology: abstract 581
Papini R (2004) Management of burn injuries of various depths. Br Med J 329:158–160
Pasyk KA, Thomas SV, Hassett CA, Cherry GW, Faller R (1989) Regional differences in capillary density of the normal human dermis. Plast Reconstr Surg 83:939–945
Salazar R, Tabernero J, Rojo F, Jimenez E, Montaner I, Casado E, Sala G, Tillner J, Malik R, Baselga J (2004) Dose-dependent inhibition of the EGFR and signalling pathways with the anti-EGFR monoclonal antibody (MAb) EMD 72000 administered every three weeks (q3w). A phase I pharmacokinetic/pharmacodynamic (PK/PD) study to define the optimal biological dose (OBD). In: Proceedings of the American Society of Clinical Oncology: abstract 2002
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbour, New York. ISBN: 0-87969-309-6
Vanhoefer U, Tewes M, Rojo F, Dirsch O, Schleucher N, Rosen O, Tillner J, Kovar A, Braun AH, Trarbach T, Seeber S, Harstrick A, Baselga J (2004) Phase I study of the humanized antiepidermal growth factor receptor monoclonal antibody EMD72000 in patients with advanced solid tumours that express the epidermal growth factor receptor. J Clin Oncol 22:175–184
Acknowledgements
Helen Gillard and Gary Coggon (Study Team Management, AstraZeneca, Alderley Park; Dinah Parums (Pathology Disease Area Clinical Expert, Experimental Medicine) and Graham Betton (Pathologist, Safety Assessment); Staff at the AstraZeneca CPU, Alderley Park. There are no conflicts of interest for any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Camidge, D., Davies, M., Laud, P. et al. Factors determining the optimal body site and method for obtaining punch biopsies of human skin as a tissue in which to assess pharmacodynamic and pharmacokinetic endpoints in drug development studies. Cancer Chemother Pharmacol 57, 52–58 (2006). https://doi.org/10.1007/s00280-005-0024-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0024-5