Abstract
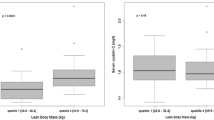
When determining the carboplatin dosage from the Calvert formula, there are a lack of data when evaluating patients with cachexia or body mass index (BMI)≥27. If the Cockcroft and Gault (C-G) creatinine clearance (CrCl) equation is utilized and substituted for glomerular filtration rate in the Calvert formula, the chance for inaccurate dosing occurs especially in these populations. Therefore, the purpose of this study is to evaluate and compare the target carboplatin area under the concentration (AUC) with the actual AUC achieved in cachectic or BMI≥27 patients. In a prospective manner, we evaluated 19 patients with a BMI≥27 and nine cachectic patients. In the C-G equation to determine creatinine clearance, the adjusted body weight was used for BMI≥27 patients and serum creatinine value of 70.7 μM (0.8 mg/dl) for the cachectic patients. The carboplatin dose was calculated, administered to the patients, and subsequent carboplatin blood samples were obtained for pharmacokinetic determination. Once the AUC was determined, the results were compared with the expected outcomes from the modified C-G CrCl equation for the Calvert formula, Chatelut and Bénézet equations. The results demonstrated that the modified C-G CrCl equation for the Calvert formula had less bias and more precision than using actual weight in the C-G CrCl equation or using the Chatelut and Bénézet equations. Using the actual weight in overweight and especially obese patients and using a serum creatinine < 70.7 μM in cachectic patients will lead to overestimation of the carboplatin clearance and thus AUC.
Similar content being viewed by others
References
Bénézet S, Guimbaud R, Chatelut E, Chevreau C, Bugat R, Canal P (1997) How to predict carboplatin clearance from standard morphological and biological characteristics in obese patients. Ann Oncol 8:607–609
Calvert AH, Newell DR, Gumbrell LA, O’Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7:1748–1756
Chatelut E, Canal P, Brunner V, Chevreau C, Pujol A, Boneu A, Roché H, Houin G, Bugat R (1995) Prediction of carboplatin clearance from standard morphological and biological patient characteristics. J Natl Cancer Inst 87:573–580
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
de Jonge ME, Mathôt RA, van Damm SM, Beijnen JH, Rodenhuis S (2002) Extremely high exposures in an obese patient receiving high-dose cyclophosphamide, thiotepa and carboplatin. Cancer Chemother Pharmacol 50:251–255
Dooley MJ, Poole SG, Rischin D, Webster LK (2002) Carboplatin dosing: gender bias and inaccurate estimates of glomerular filtration rate. Eur J Cancer 38:44–51
Dooley MJ, Singh S, Rischin D (2004) Rounding of low serum creatinine levels and consequent impact on accuracy of bedside estimates of renal function in cancer patients. Br J Cancer 90:991–995
Gore M, Mainwaring P, A’Hern R, MacFarlane V, Slevin M, Harper P, Osborne R, Mansi J, Blake P, Wiltshaw E, Shepherd J (1998) Randomized trial of dose-intensity with single-agent carboplatin in patients with epithelial ovarian cancer. London Gynaecological Group. J Clin Oncol 16:2426–2434
Hainsworth JD, Urba WJ, Hon JK, Thompson KA, Stagg MP, Hopkins LG, Thomas M, Greco FA (1998) One-hour paclitaxel plus carboplatin in the treatment of advanced non-small cell lung cancer: results of a multicentre, phase II trial. Eur J Cancer 34:654–658
Hutson PR, Tutsch KD, Pomplun M, Robins HI, Tiggelaar CL, Alberti DB, Feierabend CA, Rieck N, Arzoomanian R, Wilding G (2000) Carboplatin dosing in obese patients (abstract 725). Proc Am Soc Clin Oncol 19:187a
Izquierdo MA, Sanchez A, Llort G, Moreno MR, Germa JR (1997) Comparison of different methods for AUC dosing of carboplatin (CBDCA) (abstract 714). Proc Am Soc Clin Oncol 16:204a
Jaudon T, Séronie-Vivien S, Chatelut E, Chanut C, Favre G (2000) Comparison of the modified Jaffe method and an enzymatic method for the measurement of serum creatinine: practical consequences of a method change in the milieu of laboratory of oncologic clinical biology. Ann Biol Clin (Paris) 58:499–504
Johansen MJ, Madden T, Mehra RC, Wood JG, Rondon G, Browne V, Newman RA, Champlin RE (1997) Phase I pharmacokinetic study of multicycle high-dose carboplatin followed by peripheral-blood stem-cell infusion in patients with cancer. J Clin Oncol 15:1481–1491
Langer CJ, Leighton JC, Comis RL, O’Dwyer PJ, McAleer CA, Bonjo CA, Engstrom PF, Litwin S, Ozols RF (1995) Paclitaxel and carboplatin in combination in the treatment of advanced non-small-cell lung cancer: a phase II toxicity, response, and survival analysis. J Clin Oncol 13:1860–1870
Léger F, Séronie-Vivien S, Makdessi J, Lochon I, Delord JP, Sarda C, Canal P, Chatelut E (2002) Impact of the biochemical assay for serum creatinine measurement on the individual carboplatin dosing: a prospective study. Eur J Cancer 38:52–56
Madden T, Sunderland M, Santana VM, Rodman JH (1992) The pharmacokinetics of high-dose carboplatin in pediatric patients with cancer. Clin Pharmacol Ther 51:701–707
Martin L, Chatelut E, Boneu A, Rostaing L, Roussilhes C, Caselles O, Canal P (1998) Improvement of the Cockcroft-Gault equation for predicting glomerular filtration in cancer patients. Bull Cancer 85:631–636
Mirahmadi MK, Byrne C, Barton C, Penera N, Gordon S, Vaziri ND (1983) Prediction of creatinine clearance from serum creatinine in spinal cord injury patients. Paraplegia 21:23–29
Okamoto H, Nagatomo A, Kunitoh H, Kunikane H, Watanabe K (1998) Prediction of carboplatin clearance calculated by patient characteristics or 24-hour creatinine clearance: a comparison of the performance of three formulae. Cancer Chemother Pharmacol 42:307–312
Roa V, Conner A, Mitchell RB (1998) Carboplatin and paclitaxel for previously treated patients with non-small-cell lung cancer. Cancer Invest 16:381–384
Robert S, Zarowitz BJ, Peterson EL, Dumler F (1993) Predictability of creatinine clearance estimates in critically ill patients. Crit Care Med 21:1487–1495
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503–512
Stiff P, Unger J, Forman S, LeBlanc M, Miller T, Press O, Thompson J, Fisher R (2004) Impact of increased body weight on acute toxicity and outcome of autologous stem cell transplantation for non-Hodgkin’s lymphoma. Blood 104:104a
Smythe M, Hoffman J, Kizy K, Dmuchowski C (1994) Estimating creatinine clearance in elderly patients with low serum creatinine concentrations. Am J Hosp Pharm 51:198–204
Tran HT, Blumenschein GR Jr, Lu C, Meyers CA, Papadimitrakopoulou V, Fossella FV, Zinner R, Madden T, Smythe LG, Puduvalli VK, Munden R, Truong M, Herbst RS (2004) Clinical and pharmacokinetic study of TNP-470, an angiogenesis inhibitor, in combination with paclitaxel and carboplatin in patients with solid tumors. Cancer Chemother Pharmacol 54:308–314
van der Vijgh WJF (1991) Clinical pharmacokinetics of carboplatin. Clin Pharmacokinet 21:242–261
van Warmerdam LJC, Rodenhuis S, ten Bokkel Huinink WW, Maes RAA, Beijnen JH (1996) Evaluation of formulas using serum creatinine level to calculate the optimal dosage of carboplatin. Cancer Chemother Pharmacol 37:266–270
Wright JG, Boddy AV, Highley M, Fenwick J, McGill A, Calvert AH (2001) Estimation of glomerular filtration rate in cancer patients. Br J Cancer 84:452–459
Wurtz R, Itokazu G, Rodvold K (1997) Antimicrobial dosing in obese patients. Clin Infect Dis 25:112–118
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Herrington, J.D., Tran, H.T. & Riggs, M.W. Prospective evaluation of carboplatin AUC dosing in patients with a BMI ≥27 or Cachexia. Cancer Chemother Pharmacol 57, 241–247 (2006). https://doi.org/10.1007/s00280-005-0012-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-005-0012-9