Abstract
Purpose
Oral administration of 9-nitrocamptothecin (9NC), and the formation of its metabolite 9-aminocamptothecin (9AC), may be associated with high interpatient and intrapatient variability. Therefore, we evaluated the plasma pharmacokinetics and urine recovery of 9NC administered on three different schedules as part of phase I and phase II studies.
Experimental design
In phase I schedule A, 9NC was administered orally daily for 5 days per week for 2 weeks repeated every 4 weeks. On phase I schedule B, 9NC was administered daily for 14 days repeated every 4 weeks. In Phase II, 9NC was administered daily for 5 days during 8 weeks (one cycle). Serial blood samples were obtained on day 1 and day 10 or 11 for phase I studies, and day 1 and day 50 for the phase II study. Recovery of 9NC and 9AC in urine was evaluated on day 1 and day 10 or 11 in the phase I study. Area under the 9NC and 9AC plasma concentration vs time curves from 0 to 24 h (AUC0–24 h) were calculated using compartmental analysis.
Results
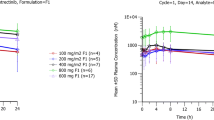
The mean±SD 9NC lactone AUC0–24 h values on day 1 at the maximum tolerated dose of schedules A and B (2.43 and 1.70 mg/m2, respectively) and the phase II dose (1.5 mg/m2) were 78.9±54.4, 155.7±112.8, and 48.3±17.5 ng/ml·h, respectively. The mean±SD 9AC lactone AUC0–24 h values at these same doses of 9NC were 17.3±17.9, 41.3±16.6, and 31.3±12.8 ng/ml h, respectively. The ratios of 9NC lactone AUC0–24 h on day 10 or 11 to day 1 on phase I A and B were 1.27±0.68 and 1.73±1.56, respectively, and the ratios 9AC lactone AUC0–24 h on day 10 or 11 to day 1 on phase I A and B were 2.23±1.02 and 1.65±0.97, respectively. The recovery of 9NC and 9AC in the urine was <15%.
Conclusions
There was significant interpatient and intrapatient variability in the disposition of 9NC and 9AC. 9NC and 9AC undergo primarily nonrenal elimination.




Similar content being viewed by others
References
Hsiang YH, Hertzberg R, Hecht S, Liu LF (1985) Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873
Covey JM, Jaxel C, Kohn KW, Pommier Y (1989) Protein-linked DNA strand breaks induced in mammalian cells by camptothecin, an inhibitor of topoisomerase I. Cancer Res 49:5016
Jaxel C, Kohn KW, Wani MC, Wall ME, Pommier Y (1989) Structure-activity study of the actions of camptothecin derivatives on mammalian topoisomerase I: evidence for a specific receptor site and a relation to antitumor activity. Cancer Res 49:1465
Kirstein MN, Houghton PJ, Cheshire PJ, Richmond LB, Smith AK, Hanna SK, Stewart CF (2001) Relation between 9-aminocamptothecin systemic exposure and tumor response in human solid tumor xenografts. Clin Cancer Res 7:358
Pazdur R, Diaz-Canton E, Ballard WP, Bradof JE, Graham S, Arbuck SG, Abbruzzese JL, Winn R (1997) Phase II trial of 9-aminocamptothecin administered as a 72-hour continuous infusion in metastatic colorectal carcinoma. J Clin Oncol 15:2905
Takimoto CH, Thomas R (2000) The clinical development of 9-aminocamptothecin. Ann N Y Acad Sci 922:224
Furman WL, Stewart CF, Poquette CA, Pratt CB, Santana VM, Zamboni WC, Bowman LC, Ma MK, Hoffer FA, Meyer WH, Pappo AS, Walter AW, Houghton PJ (1999) Direct translation of a protracted irinotecan schedule from a xenograft model to a phase I trial in children. J Clin Oncol 17:1815
Thompson J, Zamboni WC, Cheshire PJ, Lutz L, Luo X, Li Y, Houghton JA, Stewart CF, Houghton PJ (1997) Efficacy of systemic administration of irinotecan against neuroblastoma xenografts. Clin Cancer Res 3:423
Hsiang YH, Lihou MG, Liu LF (1989) Arrest of replication forks by drug-stabilized topoisomerase I-DNA cleavable complexes as a mechanism of cell killing by camptothecin. Cancer Res 49:5077
Del Bino G, Darzynkiewicz Z (1991) Camptothecin, teniposide, or 4′-(9-acridinylamino)-3-methanesulfon-m-anisidide, but not mitoxantrone or doxorubicin, induces degradation of nuclear DNA in the S phase of HL-60 cells. Cancer Res 51:1165
Houghton PJ, Cheshire PJ, Hallman JD, Lutz L, Friedman HS, Danks MK, Houghton JA (1995) Efficacy of topoisomerase I inhibitors, topotecan and irinotecan, administered at low dose levels in protracted schedules to mice bearing xenografts of human tumors. Cancer Chemother Pharmacol 36:393
Zamboni WC, Bowman LC, Tan M, Santana VM, Houghton PJ, Meyer WH, Pratt CB, Heideman RL, Gajjar AJ, Pappo AS, Stewart CF (1999) Interpatient variability in bioavailability of the intravenous formulation of topotecan given orally to children with recurrent solid tumors. Cancer Chemother Pharmacol 43:454
Gupta E, Luo F, Lallo A, Ramanathan S, Vyas V, Rubin E, Sinko P (2000) The intestinal absorption of camptothecin, a highly lipophilic drug, across Caco-2 cells is mediated by active transporter(s). Anticancer Res 20:1013
Drengler RL, Kuhn JG, Schaaf LJ, Rodriguez GI, Villalona-Calero MA, Hammond LA, Stephenson JA Jr, Hodges S, Kraynak MA, Staton BA, Elfring GL, Locker PK, Miller LL, Von Hoff DD, Rothenberg ML (1999) Phase I and pharmacokinetic trial of oral irinotecan administered daily for 5 days every 3 weeks in patients with solid tumors. J Clin Oncol 17:685
Loos WJ, Gelderblom H, Sparreboom A, Verweij J, de Jonge MJ (2000) Inter- and intrapatient variability in oral topotecan pharmacokinetics: implications for body-surface area dosage regimens. Clin Cancer Res 6:2685
Kruijtzer CM, Beijnen JH, Rosing H, Bokkel Huinink WW, Schot M, Jewell RC, Paul EM, Schellens JH (2002) Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol 20:2943
Akhtar S, Beckman RA, Mould DR, Doyle E, Fields SZ, Wright J (2000) Pretreatment with ranitidine does not reduce the bioavailability of orally administered topotecan. Cancer Chemother Pharmacol 46:204
Mi Z, Burke TG (1994) Differential interactions of camptothecin lactone and carboxylate forms with human blood components. Biochemistry 33:10325
Mi Z, Burke TG (1994) Marked interspecies variations concerning the interactions of camptothecin with serum albumins: a frequency-domain fluorescence spectroscopic study. Biochemistry 33:12540
Schrijvers D, Highley M, De Bruyn E, Van Oosterom AT, Vermorken JB (1999) Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Anticancer Drugs 10:147
Schoemaker NE, Mathot RAA, Schoffski P, Rosing H, Schellens JHM, Beijnen JH (2002) Development of an optimal pharmacokinetic sampling schedule for rubitecan administered orally in a daily times five schedule. Cancer Chemother Pharmacol 50:514–517
Raymond D, Campone M, Stupp R, Menten J, Chollet P, Lesimple T, Fety-Deporte R, Lacombe D, Paoletti X, Fumoleau P (2002) Multicentre phase II and pharmacokinetic study of RFS2000 (9-nitrocamptothecin) administered orally 5 days a week in patients with glioblastoma multiforme. Eur J Cancer 38:1348–1350
Schoffski P, Herr A, Vermorken JB, Van den BJ, Beijnen JH, Rosing H, Volk J, Ganser A, Adank S, Botma HJ, Wanders J (2002) Clinical phase II study and pharmacological evaluation of rubitecan in non-pretreated patients with metastatic colorectal cancer—significant effect of food intake on the bioavailability of the oral camptothecin analogue. Eur J Cancer 38:807
Natelson EA, Giovanella BC, Verschraegen CF, Fehir KM, De Ipolyi PD, Harris N, Stehlin JS (1996) Phase I clinical and pharmacological studies of 20-(S)-camptothecin and 20-(S)-9-nitrocamptothecin as anticancer agents. Ann N Y Acad Sci 803:224
Zamboni WC, Jung LL, Egorin MJ, Potter DM, Friedland DM, Belani CP, Agarwala SS, Wong MM, Fakih M, Trump DL, Jin R, Strychor S, Vozniak M, Troetschel M, Ramanathan RK (2004) Phase I and pharmacologic study of intermittently administered 9-nitrocamptothecin in patients with advanced solid tumors. Clin Cancer Res 10:5058
Potter DM (2002) Adaptive dose finding for phase I clinical trials of drugs used for chemotherapy of cancer. Stat Med 21:1805
O’Quigley J, Pepe M, Fisher L (1990) Continual reassessment method: a practical design for phase 1 clinical trials in cancer. Biometrics 46:33
Supko JG, Malspeis L (1992) Liquid chromatographic analysis of 9-aminocamptothecin in plasma monitored by fluorescence induced upon post-column acidification. J Liquid Chromatogr 15:3261
D’Argenio DZ, Schmuitzky A (1997) ADAPT II user’s guide. Biomedical Simulation Resource, University of Southern California, Los Angeles
Rowland M, Tozer T (eds) (1999) Clinical pharmacokinetics: concepts and applications. Lea and Febiger, Philadelphia
Verschraegen CF, Gilbert BE, Huaringa AJ, Newman R, Harris N, Leyva FJ, Keus L, Campbell K, Nelson-Taylor T, Knight V (2000) Feasibility, phase I, and pharmacological study of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced malignancies in the lungs. Ann N Y Acad Sci 922:352
Gilbert BE, Newman R, Zamboni WC, Knight V, Verschraegen CF (2001) Pharmacokinetics of multiple 9-nitro-camptothecin (9NC) liposome aerosols during a Phase I study: levels of total and lactone forms, and its conversion to 9-amino-camptothecin (9AC). Proc Am Soc Clin Onc 20:90b
Gounder M, Saleem A, Roychowdhury M (2002) Metabolism of 9-nitrocamptothecin (RFS2000/9NC) to 9-aminocamptothecin (9AC) in patients and in vitro. Proc Am Assoc Cancer Res 42
Pantazis P, Harris N, Mendoza J, Giovanella B (1995) The role of pH and serum albumin in the metabolic conversion of 9-nitrocamptothecin to 9-aminocamptothecin by human hematopoietic and other cells. Eur J Haematol 55:211
Hinz HR, Harris NJ, Natelson EA, Giovanella BC (1994) Pharmacokinetics of the in vivo and in vitro conversion of 9-nitro-20(S)-camptothecin to 9-amino-20(S)-camptothecin in humans, dogs, and mice. Cancer Res 54:3096
Acknowledgements
Supported, in part, by grant NCI 2P30 CA47904, grant NIH/NCRR/GCRC/#5M01RR00056, and a grant from SuperGen, Inc., Dublin, California.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jung, L.L., Ramanathan, R.K., Egorin, M.J. et al. Pharmacokinetic studies of 9-nitrocamptothecin on intermittent and continuous schedules of administration in patients with solid tumors. Cancer Chemother Pharmacol 54, 487–496 (2004). https://doi.org/10.1007/s00280-004-0835-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0835-9