Abstract
Background
Oxaliplatin and topotecan are novel options for a variety of neoplasms. Topotecan has shown fewer side effects and higher efficacy when given as a continuous i.v. infusion compared to single doses, but this regimen has not yet been combined with oxaliplatin.
Patients and methods
This phase I/II trial was designed to establish the dose-limiting toxicity of a combination of oxaliplatin (85–130 mg/m2 on day 1) and a continuous infusion of topotecan (initial 0.9 mg/m2 over 72–120 h). Eligible patients with metastatic colorectal cancer had progressive disease during, or within 12 weeks after, palliative fluoropyrimidine-based chemotherapy or in whom intolerable 5-FU toxicity had developed.
Results
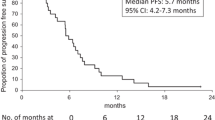
The study included 21 patients. Subjectively the treatment was well tolerated but haematological toxicity was observed with the initial treatment schedule of oxaliplatin 85 mg/m2 on day 1 and topotecan 0.9 mg/m2 on days 1–5. Reducing topotecan to 0.9 mg/m2 on days 1–3 resulted also in acceptable haematological toxicity. In patients completing three or more therapy cycles, median progression-free survival was 5 months, and 50% had stable disease or showed a partial response.
Conclusion
The recommended dose of this combination for further testing is oxaliplatin 85 mg/m2 on day 1 and topotecan 0.9 mg/m2 per day as a continuous infusion on days 1–3.

Similar content being viewed by others
References
Alexandre J, Tigaud JM, Gross-Goupil M, et al (2002) Combination of toptecan and oxaliplatin in inoperable hepatocellular cancer patients. Am J Clin Oncol 25:198–203
Burris HA III, Awada A, Kuhn JG, et al (1994) Phase I and pharmacokinetic studies of topotecan administered as 72 and 120 h continuous infusion. Anticancer Drugs 5:394–402
Burris III HA, Hanauske AR, Johnsohn RK et al (1992) Activity of topotecan, a new topoisomerase I inhibitor, against human tumour colony-forming units. J Natl Cancer Inst 84:1816–1820
Carmichael J, Ozols RF (1997) Topotecan, an active new antineoplastic agent: review and current status. Expert Opin Investig Drugs 6:593–608
Conti JA, Kemeny NE, Saltz LB, et al (1996) Irinotecan is an active agent in untreated patients with metastatic colorectal cancer. J Clin Oncol 14:709–715
Creemers GJ (1997) Topotecan in advanced colorectal cancer. Semin Oncol 24:S20–S48
Desch CE, Benson AB 3rd, Smith TJ, et al (1999) Recommended colorectal cancer surveillance guidelines by the American society of clinical oncology. J Clin Oncol 17:1312–1321
Giacchetti S, Perpoint B, Zidani R, Le Bail N, et al (2000) Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol 18:136–147
Goldwasser F, Bozec L, Zeghari-Squalli N, Misset JL (1999) Cellular pharmacology of the combination of oxaliplatin with topotecan in the IGROV-1 human ovarian cancer cell line. Anticancer Drugs 10:201–295
Goldwasser F, Valenti M, Torres R, Kohn KW, Pommier Y (1996) Potentiation of cisplatin cytotoxicity by 9-aminocamptothecin. Clin Cancer Res 2:687–693
Gross-Goupil M, Lobiec F, Lopez G, et al (2002) Topotecan preceded by oxaliplatin using a 3 week schedule: a phase I study in advanced cancer patients. Eur J Cancer 38:1888–1898
Hoff DD von, Burris HA III, et al (1994) Preclinical and phase I trials of topoisomerase I inhibitors. Pharmacology [Suppl] 34:41–45
Houghton PJ, Cheshire PJ, Myers, L et al (1992) Evaluation of 9-dimethylaminomethyl-10-hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol 31:229–239
Kollmansberger C, Mross K, Jakob A, Kanz L, Bokemeyer C (1999) Topotecan—a novel topoisomersae I inhibitor: pharmacology and clinical experience. Oncology 56:1–12
Lu JM, Hochster J, et al (2003) Phase I study of oxaliplatin and topotecan infusion in patients with previously treated ovarian cancer. Proc Am Soc Clin Oncol 22:abstract 1860
Machover D, Diaz-Rubio F, de Gramont A, et al (1996) Two consecutive phase II studies of oxaliplatin (L-OHP) for treatment of patients with advanced colorectal carcinoma who were resistant to previous fluoropyrimidines. Ann Oncol 7:95–98
Misset JL (1997) Chemotherapy of advanced colorectal cancers after failure of a treatment with fluoropyrimidine. Rev Prat [Suppl] 47:529–535
Pratt C, Stewart C, Santana V, et al (1994) Phase I study of topotecan for pediatric patients with malignant solid tumours. J Clin Oncol 12:539–543
Rowinsky EK, Adjei A, Donehower RC, et al (1994) Phase I and pharmacodynamik study of the topoisomerase I inhibitor topotecan in patients with refractory acute leukemia. J Clin Oncol 12:2193–2203
Saltz LB, Cox JV, Blanke C, et al (2000) Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 343:905–914
Slichenmyer WJ, Chen TL, et al (1994) Clinical pharmacology of topotecan in cancer patients with renal or hepatic dysfunction. Proc Am Soc Clin Oncol 13:abstract 363
Tortora G, Ciardiello F, Damiano V, et al (2002) Preclincal and phase I study of oxaliplatin and topotecan in combination in human cancer. Ann Oncol 13:392–398
Von Hoff DD, Rothenberg ML, Pitot HC, et al (1997) Irinotecan (CPT-11) therapy for patients with previously treated metastatic colorectal cancer (CRC): overall results of FDA-reviewed pivotal US clinical trials. Proc Am Soc Clin Oncol 16(228a):803
Author information
Authors and Affiliations
Corresponding author
Additional information
Dr. Hubert Szélenyi, born 11 January 1965, died in the prime of life on 29 August 2002. We are deeply saddened by the loss of an excellent physician, accomplished scientist and admirable and warm-hearted friend and colleague. He had essentially designed and developed this study, and we decided to finish it in compliance with his intentions.
Rights and permissions
About this article
Cite this article
Hütter, G., Szélenyi, H., Deckert, P.M. et al. Phase I/II trial of topotecan given as continuous infusion in combination with oxaliplatin in 5-FU-pretreated patients with colorectal cancer. Cancer Chemother Pharmacol 54, 178–184 (2004). https://doi.org/10.1007/s00280-004-0796-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-004-0796-z