Abstract
Purpose
To use a population approach to identify readily available clinical or biochemical characteristics that influence the pharmacokinetics of epirubicin and to develop new dosage guidelines based on these results.
Methods
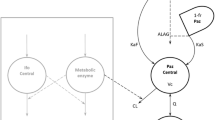
Data were available from 109 patients with advanced breast cancer, 72 of whom were known to have liver metastases. They were treated with single-agent epirubicin 12.5 to 120 mg/m2. Analysis was performed using the software package NONMEM and a three-compartment model was fitted to the data.
Results
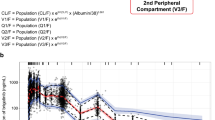
Individual clearance (CL) estimates ranged from 4 to 86 l/h and the final model included CL as a function of aspartate aminotransferase (AST): CL (l/h)=72.9−(72.9×0.135×lnAST). Inclusion of this factor reduced the interindividual variability in CL from 49% to 39%. Using a target AUC of 4000 ng·h/ml, the following doses were predicted to achieve this exposure with the greatest precision: AST <150 IU/l 125 mg; AST 150–250 IU/l 90 mg; AST 250–500 IU/l 60 mg; AST >500 IU/l 30 mg. These new guidelines were compared with three other guidelines based on serum bilirubin or AST concentrations and body surface area (BSA). The new guidelines achieved the target with greater precision (root mean squared error, rmse, 39.0%) than the current UK guidelines, current USA guidelines or an earlier equation based on AST (rmse 63%, 62% and 59%, respectively).
Conclusions
The proposed dosing guidelines should reduce variability in systemic exposure to epirubicin more effectively than traditional approaches. In addition, as they do not require adjustment according to BSA, they could reduce dosage preparation time and the potential for prescribing and dispensing errors.



Similar content being viewed by others
References
Beal SL, Sheiner LB (1992) NONMEM (user's guide), parts 1-VII. Technical Report, University of California, San Francisco
Camaggi CM, Strocchi E, Tamassia V, Martoni A, Giovannini M, Iafelice G, Canova N, Marraro D, Martini A, Pannuti F (1982) Pharmacokinetic studies of 4′-epi-doxorubicin in cancer patients with normal and impaired renal function and with liver metastases. Cancer Treat Rep 66:1819
Cook RD (1977) Detection of influential observation in linear regression. Technometrics 19:15
Coukell AJ, Faulds D (1997) Epirubicin. An updated review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of breast cancer. Drugs 53:453
Dobbs NA, Twelves CJ (1998) What is the effect of adjusting epirubicin doses for body surface area? Br J Cancer 78:662
Dobbs NA, Twelves CJ (1991) Measurement of epidoxorubicin and its metabolites by high-performance liquid chromatography using an advanced automated sample processor. J Chromatogr 572:211
Dobbs NA, Twelves CJ, Gregory W, Cruickshanka C, Richards MA, Rubens RD (2003) Epirubicin in patients with liver dysfunction: development and evaluation of a novel dose modification scheme. Eur J Cancer 39:580–586
Epirubicin Product Label, UK (2003) http://emc.vhn.net
Epirubicin Product Label, USA (2003) www.fda.gov/cder/foi/label/1999/50778lbl.pdf
Favier M, deCazanove F, Saint Martin F, Bressolle F (1994) Preventing medication errors in antineoplastic therapy. Am J Hosp Pharm 51:832
Grochow LB, Baraldi C, Noe D (1990) Is dose normalization to weight or body surface area useful in adults? J Natl Cancer Inst 82:323
Gurney HP, Ackland S, Gebski V, Farrell G (1998) Factors affecting epirubicin pharmacokinetics and toxicity: evidence against using body-surface area for dose calculation. J Clin Oncol 16:2299
Jakobsen P, Basholt L, Dalmark M, Pfeiffer P, Petersen D, Gjedde SB, Sandberg E, Rose C, Nielsen OS, Mouridsen HT (1991) A randomised study of epirubicin at four different dose levels in advanced breast cancer. Feasibility of myelotoxicity prediction through single blood-sample measurement. Cancer Chemother Pharmacol 28:465
Jonsson EN, Karlsson MO (1999) Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Methods Programs Biomed 58:51
Plosker GL, Faulds D (1993) Epirubicin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in cancer chemotherapy. Drugs 45:788
Robert J (1994) Clinical pharmacokinetics of epirubicin. Clin Pharmacokinet 26:428
Robert J (1993) Epirubicin. Clinical pharmacology and dose-effect relationship. Drugs 45:20
Rodvold KA, Rushing DA, Tewksbury DA (1988) Doxorubicin clearance in the obese. J Clin Oncol 6:1321
Sheiner LB, Beal SL (1980) Evaluation of methods for estimating population pharmacokinetic parameters I. Michaelis-Menten model: routine clinical pharmacokinetic data. J Pharmacokinet Biopharm 8:553
Sheiner LB, Beal SL (1981) Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm 9:503
Twelves CJ, Dobbs NA, Michael Y, Summers LA, Gregory W, Harper PG, Rubens RD, Richards MA (1992) Clinical pharmacokinetics of epirubicin: the importance of liver biochemistry tests. Br J Cancer 66:765
Weenan H, Lankelma H, Penders PGM, McVie JG, Ten Bokkel Huinink WW, de Planque MM, Pinedo HM (1983) Pharmacokinetics of 4′-epi-doxorubicin in man. Invest New Drugs 1:59
Acknowledgement
The authors would like to thank Quintiles Ltd for supporting Lorraine Ralph.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ralph, L.D., Thomson, A.H., Dobbs, N.A. et al. A population model of epirubicin pharmacokinetics and application to dosage guidelines. Cancer Chemother Pharmacol 52, 34–40 (2003). https://doi.org/10.1007/s00280-003-0608-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0608-x