Abstract
Purpose
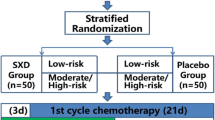
Kampo medicine Hangeshashin-to (TJ-14) which contains baicalin, a β-glucuronidase inhibitor, alleviates diarrhea induced by irinotecan (CPT-11). We conducted a randomized comparative trial to investigate whether support with TJ-14 would prevent and control CPT-11-induced diarrhea.
Methods
Of 44 previously untreated patients with advanced non-small-cell lung cancer randomized, 41 (18 TJ-14 group, 23 control group) were available for evaluation. The chemotherapy regimen consisted of a combination of cisplatin and CPT-11. TJ-14 (7.5 g/day) was administered orally.
Results
Of the 41 patients, 39 experienced diarrhea. Compared with the control group, the TJ-14 group showed a significant improvement in diarrhea grades (P=0.044) as well as a reduced frequency of diarrhea grades 3 and 4 (one patient versus ten patients; P=0.018). However, the two groups showed no differences in the frequency of diarrhea or the number of days the symptoms continued. This study was stopped at an interim evaluation.
Conclusion
TJ-14 was effective in preventing and controlling CPT-11-induced diarrhea.
Similar content being viewed by others
References
Abigerges D, Armand J-P, Chabot GG, Costa LD, Fadel E, Cote C, Herait D, Gandia D (1994) Irinotecan (CPT-11) high-dose escalation using intensive high-dose loperamide to control diarrhea. J Natl Cancer Inst 86:446
Asumi R, Suzuki W, Hakusui H (1991) Identification of the metabolites of irinotecan, a new derivative of camptothecin, in rat bile and its biliary excretion. Xenobiotica 21:1159
Fukuoka M, Masuda N, Furuse K, Negoro S, Takada M, Matsui K, Takifuji N, Kudoh S, Kawahara M, Ogawara M, Kodama N, Kubota K, Yamamoto M, Kusunoki Y (1991) A randomized trial in inoperable non-small-cell lung cancer: vindesine and cisplatin versus mitomycin, vindesine and cisplatin versus etoposide and cisplatin alternating with vindesine and mitomycin. J Clin Oncol 9:606
Fukuoka M, Niitani H, Suzuki A, Motomiya M, Hasegawa K, Nishiwaki Y, Kuriyama T, Ariyoshi Y, Negoro S, Masuda N, Nakajima S, Taguchi T (1992) A phase II study of CPT-11, a new derivative of untreated non-small-cell lung cancer. J Clin Oncol 10:16
Gandia D, Abigerges D, Armand J-P, Chabot G, Costa LD, Forni MD (1993) CPT-11 induced cholinergic effects in cancer patients (letter). J Clin Oncol 11:196
Kaneda N, Nagata H, Furuta T, Yokokura T (1990) Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in mouse. Cancer Res 50:1715
Kunimoto T, Nitta K, Tanaka T, Uehara N, Baba H, Takeuchi M, Yokokura T, Sawada S, Miyasaka T, Mutai M (1987) Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxy-camptothecin, a novel water soluble derivative of camptothecin, against murine tumors. Cancer Res 47:5944
Masuda N, Fukuoka M, Kusunoki Y, Mastui K, Takifuji N, Kudoh S, Negoro S, Nishioka M, Nakagawa K, Takada M (1992) CPT-11: a new derivative of camptothecin, for the treatment of refractory or relapsed small-cell lung cancer. J Clin Oncol 10:1225
Matsuzaki T, Yokokura T, Mutai M, Tsuruo T (1988) Inhibition of spontaneous and experimental metastasis by a new derivative of camptothecin, CPT-11, in mice. Cancer Chemother Pharmacol 21:308
Mori K, Saito Y, Tominaga K (1992) Phase II study of cisplatin continuous infusion plus vindesine in the treatment of non-small-cell lung cancer. Am J Clin Oncol 15:344
Mori K, Hirose T, Machida S, Yokoyama K, Tominaga K (1997) A phase I study of irinotecan and infusional cisplatin with recombinant human granulocyte colony-stimulating factor support in the treatment of advanced non-small cell lung cancer. Eur J Cancer 33:503
Narita M, Nagai E, Hagiwara H, Aburada M, Yokoi T, Kamataki T (1993) Inhibition of β-glucuronidase by natural glucuronides of Kampo medicines using glucuronide of SN-38 (7-ethyl-10-hydroxycamptothecin) as a substrate. Xenobiotica 23:5
Negoro S, Fukuoka N, Masuda N, Takada M, Kusunoki Y, Matsui K, Takifuji N, Kudoh S, Niitani H, Taguchi T (1991) Phase I study of weekly administration of CPT-11. J Natl Cancer Inst 83:1164
Noda K, Nishiwaki Y, Kawahara M, Negoro S, Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, Yamamoto S, Saijo N (2002) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85
Ohno R, Okada K, Masaoka T, Kuramoto A, Arima T, Yoshida Y, Ariyoshi H, Ichimaru M, Sakai Y, Oguro M, Ito Y, Morishima Y, Yokomaku S, Ota K (1990) An early phase II study of CPT-11: a new derivative of camptothecin, for the treatment of leukemia and lymphoma. J Clin Oncol 8:1907
Oshita F, Noda K, Nishiwaki Y, Fujita A, Kurita Y, Nakabayashi T, Tobise K, Abe S, Suzuki S, Hayashi I, Kawakami Y, Matsuda T, Tsuchiya S, Takahashi S, Tamura T, Saijo N (1997) Phase II study of irinotecan and etoposide in patients with metastatic non-small-cell lung cancer. J Clin Oncol 15:304
Rothenberg ML, Kuhn JG, Burris HA III, Nelson J, Eckardt JR, Tristan-Morales M, Hilsenbeck SG, Weiss GR, Smith LS, Rodriguez Gl, Rock MK, Von Hoff DD (1993) Phase I and pharmacokinetic trial of weekly CPT-11. J Clin Oncol 11:2194
Sakata Y, Suzuki H, Kamataki T (1994) Preventive effect of TJ-14, a Kampo (Chinese herb) medicine, on diarrhea induced by irinotecan hydrochloride (CPT-11) (in Japanese with English abstract). Jpn J Cancer Chemother 21:1241
Shimada Y, Yoshino M, Wakui A, Nakao I, Futatsuki K, Sakata Y, Kambe M, Taguchi T, Ogawa N (1993) Phase II study of CPT-11, a new camptothecin derivative, in metastatic colorectal cancer. J Clin Oncol 11:909
Takasuna K, Kasai Y, Kitano Y, Mori K, Kobayashi R, Hagiwara T, Kakihata K, Hirohashi M, Nomura M, Nagai E, Kamataki T (1995) Protective effects of Kampo medicine and baicaline against intestinal toxicity of a new anticancer camptothecin derivative, irinotecan hydrochloride (CPT-11), in rats. Jpn J Cancer Res 86:978
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported in part by a Grant-in Aid for Cancer Research from the Ministry of Health, Labour and Welfare, the Foundation for the Promotion of Cancer Research, and the Second Term Comprehensive 10-Year Strategy for Cancer Control.
Rights and permissions
About this article
Cite this article
Mori, K., Kondo, T., Kamiyama, Y. et al. Preventive effect of Kampo medicine (Hangeshashin-to) against irinotecan-induced diarrhea in advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 51, 403–406 (2003). https://doi.org/10.1007/s00280-003-0585-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0585-0