Abstract
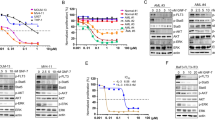
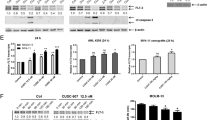
De novo acute myeloid leukemia (AML) patients with FMS-like tyrosine kinase 3 internal tandem duplications (FLT3-ITD) have worse treatment outcomes. Arsenic trioxide (ATO) used in the treatment of acute promyelocytic leukemia (APL) has been reported to be effective in degrading the FLT3 protein in AML cell lines and sensitizing non-APL AML patient samples in-vitro. We have previously reported that primary cells from FLT3-ITD mutated AML patients were sensitive to ATO in-vitro compared to other non-M3 AML and molecular/pharmacological inhibition of NF-E2 related factor 2 (NRF2), a master regulator of antioxidant response improved the chemosensitivity to ATO and daunorubicin even in non FLT3-ITD mutated cell lines and primary samples. We examined the effects of molecular/pharmacological suppression of NRF2 on acquired ATO resistance in the FLT3-ITD mutant AML cell line (MV4-11-ATO-R). ATO-R cells showed increased NRF2 expression, nuclear localization, and upregulation of bonafide NRF2 targets. Molecular inhibition of NRF2 in this resistant cell line improved ATO sensitivity in vitro. Digoxin treatment lowered p-AKT expression, abrogating nuclear NRF2 localization and sensitizing cells to ATO. However, digoxin and ATO did not sensitize non-ITD AML cell line THP1 with high NRF2 expression. Digoxin decreased leukemic burden and prolonged survival in MV4-11 ATO-R xenograft mice. We establish that altering NRF2 expression may reverse acquired ATO resistance in FLT3-ITD AML.




Similar content being viewed by others
Data availability
Not applicable
References
George B et al (2004) Treatment of children with newly diagnosed acute promyelocytic leukemia with arsenic trioxide: a single center experience. Leukemia 18:1587–1590
Mathews V et al (2006) Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: durable remissions with minimal toxicity. Blood 107:2627–2632
Mathews V et al (2002) Arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: a single center experience. Am J Hematol 70:292–299
Mathews V et al (2010) Single-agent arsenic trioxide in the treatment of newly diagnosed acute promyelocytic leukemia: long-term follow-up data. J Clin Oncol Off J Am Soc Clin Oncol 28:3866–3871
Hu J et al (1999) Long-term survival and prognostic study in acute promyelocytic leukemia treated with all-trans-retinoic acid, chemotherapy, and As2O3: an experience of 120 patients at a single institution. Int J Hematol 70:248–260
Soignet SL et al (1998) Complete Remission after Treatment of Acute Promyelocytic Leukemia with Arsenic Trioxide. N Engl J Med 339:1341–1348
Niu H et al (2017) Endogenous Retinoid X Receptor ligands in mouse hematopoietic cells. Sci Signal 10:eaan1011
Zhou J et al (2010) Single-agent arsenic trioxide in the treatment of children with newly diagnosed acute promyelocytic leukemia. Blood 115:1697–1702
Parmar S et al (2004) Phase II trial of arsenic trioxide in relapsed and refractory acute myeloid leukemia, secondary leukemia and/or newly diagnosed patients at least 65 years old. Leuk Res 28:909–919
Burnett AK et al (2011) The addition of arsenic trioxide to low-dose Ara-C in older patients with AML does not improve outcome. Leukemia 25:1122–1127
Welch JS et al (2011) Combination decitabine, arsenic trioxide, and ascorbic acid for the treatment of myelodysplastic syndrome and acute myeloid leukemia: a phase I study. Am J Hematol 86:796–800
Rojewski MT, Baldus C, Knauf W, Thiel E, Schrezenmeier H (2002) Dual effects of arsenic trioxide (As2O3) on non-acute promyelocytic leukaemia myeloid cell lines: induction of apoptosis and inhibition of proliferation. Br J Haematol 116:555–563
Noguera NI et al (2017) High-dose ascorbate and arsenic trioxide selectively kill acute myeloid leukemia and acute promyelocytic leukemia blasts in vitro. Oncotarget 8:32550–32565
Abraham A et al (2014) ABCB6 RNA expression in leukemias–expression is low in acute promyelocytic leukemia and FLT3-ITD-positive acute myeloid leukemia. Ann Hematol 93:509–512
Varatharajan S et al (2017) ATP-binding casette transporter expression in acute myeloid leukemia: association with in vitro cytotoxicity and prognostic markers. Pharmacogenomics 18:235–244
Nagai K et al (2018) Combination of ATO with FLT3 TKIs eliminates FLT3/ITD+ leukemia cells through reduced expression of FLT3. Oncotarget 9:32885–32899
Liang C et al (2020) Arsenic trioxide and all-trans retinoic acid suppress the expression of FLT3-ITD. Leuk Lymphoma 61:2692–2699
Liu X-J et al (2020) Arsenic trioxide induces autophagic degradation of the FLT3-ITD mutated protein in FLT3-ITD acute myeloid leukemia cells. J Cancer 11:3476–3482
Karathedath S et al (2017) Molecular and Pharmacological Inhibition of ATP Binding Cassette Transporter ABCB6 Decreases Chemoresistance in Acute Myeloid Leukemia. Blood 130:1254
Liu X et al (2023) Targeting NRF2 uncovered an intrinsic susceptibility of acute myeloid leukemia cells to ferroptosis. Exp Hematol Oncol 12:47
Menegon S, Columbano A, Giordano S (2016) The Dual Roles of NRF2 in Cancer. Trends Mol Med 22:578–593
Rojo de la Vega M, Chapman E, Zhang DD (2018) NRF2 and the Hallmarks of Cancer. Cancer Cell 34:21–43
Wu S, Lu H, Bai Y (2019) Nrf2 in cancers: A double-edged sword. Cancer Med 8:2252–2267
Wang X-J et al (2008) Nrf2 enhances resistance of cancer cells to chemotherapeutic drugs, the dark side of Nrf2. Carcinogenesis 29:1235–1243
Lin P et al (2019) The high NRF2 expression confers chemotherapy resistance partly through up-regulated DUSP1 in myelodysplastic syndromes. Haematologica 104:485–496
Kim HG et al (2019) Quinacrine-Mediated Inhibition of Nrf2 Reverses Hypoxia-Induced 5-Fluorouracil Resistance in Colorectal Cancer. Int J Mol Sci 20:4366
Sun Y, Abdul Aziz A, Bowles K, Rushworth S (2018) High NRF2 expression controls endoplasmic reticulum stress induced apoptosis in multiple myeloma. Cancer Lett 412:37–45
Xiang Y et al (2018) Brusatol Enhances the Chemotherapy Efficacy of Gemcitabine in Pancreatic Cancer via the Nrf2 Signalling Pathway. Oxid Med Cell Longev 2018:2360427
Khamari R et al (2018) Glucose metabolism and NRF2 coordinate the antioxidant response in melanoma resistant to MAPK inhibitors. Cell Death Dis 9:325
Kitamura H, Motohashi H (2018) NRF2 addiction in cancer cells. Cancer Sci 109:900–911
Shin D, Kim EH, Lee J, Roh J-L (2018) Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med 129:454–462
Silva MM, Rocha CRR, Kinker GS, Pelegrini AL, Menck CFM (2019) The balance between NRF2/GSH antioxidant mediated pathway and DNA repair modulates cisplatin resistance in lung cancer cells. Sci Rep 9:17639
Singh A et al (2021) NRF2 Activation Promotes Aggressive Lung Cancer and Associates with Poor Clinical Outcomes. Clin. Cancer Res. Off J Am Assoc Cancer Res 27:877–888
Cheng C et al (2021) Inhibition of Nrf2-mediated glucose metabolism by brusatol synergistically sensitizes acute myeloid leukemia to Ara-C. Biomed Pharmacother Biomedecine Pharmacother 142:111652
Endo S et al (2021) Targeting Nrf2-antioxidant signalling reverses acquired cabazitaxel resistance in prostate cancer cells. J Biochem (Tokyo) 170:89–96
Küper A et al (2021) Overcoming hypoxia-induced resistance of pancreatic and lung tumor cells by disrupting the PERK-NRF2-HIF-axis. Cell Death Dis 12:82
Sivinski J, Zhang DD, Chapman E (2021) Targeting NRF2 to treat cancer. Semin Cancer Biol 76:61–73
Kumar H, Kumar RM, Bhattacharjee D, Somanna P, Jain V (2022) Role of Nrf2 Signaling Cascade in Breast Cancer: Strategies and Treatment. Front Pharmacol 13:720076
Kannan S et al (2022) Targeting the NRF2/HO-1 Antioxidant Pathway in FLT3-ITD-Positive AML Enhances Therapy Efficacy. Antioxid Basel Switz 11:717
Zhong Y et al (2013) Drug resistance associates with activation of Nrf2 in MCF-7/DOX cells, and wogonin reverses it by down-regulating Nrf2-mediated cellular defense response. Mol Carcinog 52:824–834
Park SH et al. (2018) Resistance to gefitinib and cross-resistance to irreversible EGFR-TKIs mediated by disruption of the Keap1-Nrf2 pathway in human lung cancer cells. FASEB J Off Publ Fed Am Soc Exp Biol. fj201800011R. https://doi.org/10.1096/fj.201800011R.
Liu Q et al (2010) The NRF2-mediated oxidative stress response pathway is associated with tumor cell resistance to arsenic trioxide across the NCI-60 panel. BMC Med Genomics 3:37
Singh A et al (2016) Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem Biol 11:3214–3225
Panieri E, Saso L (2019) Potential Applications of NRF2 Inhibitors in Cancer Therapy. Oxid Med Cell Longev 2019:8592348
Zhou Y et al (2020) Flumethasone enhances the efficacy of chemotherapeutic drugs in lung cancer by inhibiting Nrf2 signaling pathway. Cancer Lett 474:94–105
Choi E-J et al (2017) A clinical drug library screen identifies clobetasol propionate as an NRF2 inhibitor with potential therapeutic efficacy in KEAP1 mutant lung cancer. Oncogene 36:5285–5295
Zhou Y et al (2019) Digoxin sensitizes gemcitabine-resistant pancreatic cancer cells to gemcitabine via inhibiting Nrf2 signaling pathway. Redox Biol 22:101131
Laverdière I et al (2018) Leukemic stem cell signatures identify novel therapeutics targeting acute myeloid leukemia. Blood Cancer J 8:52
Illangeswaran RSS et al (2023) Chemotherapeutic drugs elicit stemness and metabolic alteration to mediate acquired drug-resistant phenotype in acute myeloid leukemia cell lines. Leuk Res 128:107054
Ianevski A, He L, Aittokallio T, Tang J (2017) SynergyFinder: a web application for analyzing drug combination dose–response matrix data. Bioinformatics 33:2413–2415
Karathedath S et al (2017) Role of NF-E2 related factor 2 (Nrf2) on chemotherapy resistance in acute myeloid leukemia (AML) and the effect of pharmacological inhibition of Nrf2. PLoS ONE 12:e0177227
Singh A et al. (2006) Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung cancer. PLoS Med 3
Hoang DH et al (2022) Arsenic Trioxide and Venetoclax Synergize against AML Progenitors by ROS Induction and Inhibition of Nrf2 Activation. Int J Mol Sci 23:6568
Ren D et al (2011) Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc Natl Acad Sci U S A 108:1433–1438
Tang X et al (2011) Luteolin inhibits Nrf2 leading to negative regulation of the Nrf2/ARE pathway and sensitization of human lung carcinoma A549 cells to therapeutic drugs. Free Radic Biol Med 50:1599–1609
Kweon M-H, Adhami VM, Lee J-S, Mukhtar H (2006) Constitutive overexpression of Nrf2-dependent heme oxygenase-1 in A549 cells contributes to resistance to apoptosis induced by epigallocatechin 3-gallate. J Biol Chem 281:33761–33772
Rada P et al (2011) SCF/{beta}-TrCP promotes glycogen synthase kinase 3-dependent degradation of the Nrf2 transcription factor in a Keap1-independent manner. Mol Cell Biol 31:1121–1133
Almazari I et al (2012) Guggulsterone induces heme oxygenase-1 expression through activation of Nrf2 in human mammary epithelial cells: PTEN as a putative target. Carcinogenesis 33:368–376
Chowdhry S et al (2013) Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32:3765–3781
Lien EC et al (2016) Glutathione biosynthesis is a metabolic vulnerability in PI(3)K/Akt-driven breast cancer. Nat Cell Biol 18:572–578
Kim N et al (2016) Cardiac glycosides display selective efficacy for STK11 mutant lung cancer. Scintific reports 6:29721
Martinez FT et al (2019) Identification and characterization of Cardiac Glycosides as senolytic compounds. Nat Commun 10:4731
Deng K et al (2019) Sodium chloride (NaCl) potentiates digoxin-induced anti-tumor activity in small cell lung cancer. Cancer Biol Ther 20:52–64
Funding
This study is supported by grants from the Department of Science and Technology, India: EMR/2017/003880 and CRG/2021/004281 to Dr. Poonkuzhali Balasubramanian.
RVS and PB are supported by Wellcome DBT India Alliance (IA/S/17/1/503118, IA/CPHS/18/1/503930, and IA/S/21/2/505932), respectively. DZP, SI, and BMR are supported by ICMR (Indian Council of Medical Research) SRF. SD and NKB are funded by the University Grants Commission and RTV by CSIR. We thank the staff of the animal facility, flow cytometry, and imaging facility of the Centre for Stem Cell Research, a unit of in Stem Bengaluru, CMC Campus, Vellore for their help.
Author information
Authors and Affiliations
Contributions
DZ, RSSI and BR designed the research, performed experiments, analyzed results, and wrote the manuscript.
RTV, SD and NB performed experiments and analyzed the results.
VM and SRV contributed to the analysis and review of the manuscript.
PB designed the research, performed experiments, analyzed results, wrote the manuscript, and procured funding.
All authors contributed to the article and approved the submitted version and revision.
Corresponding author
Ethics declarations
Competing interest
None of the authors have any competing interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jebanesan, D.Z.P., Illangeswaran, R.S.S., Rajamani, B.M. et al. Inhibition of NRF2 signaling overcomes acquired resistance to arsenic trioxide in FLT3-mutated Acute Myeloid Leukemia. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05742-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05742-8