Abstract
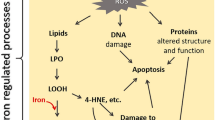
Multiple myeloma (MM) is a common malignant hematologic neoplasm, and the involvement of epigenetic modifications in its development and drug resistance has received widespread attention. Ferroptosis, a new ferroptosis-dependent programmed death mode, is closely associated with the development of MM. The novel methyltransferase inhibitor DCG066 has higher cell activity, but its mechanism of action in MM has not been clarified. Here, we found that DCG066 (5µM) inhibited the proliferation and induced ferroptosis in MM cells; the intracellular levels of ROS, iron, and MDA were significantly elevated, and the level of GSH was reduced after the treatment of DCG066; The protein expression levels of SLC7A11, GPX4, Nrf2 and HO-1 were significantly reduced, and these phenomena could be reversed by ferroptosis inhibitor Ferrostatin-1 (Fer-1) and Nrf2 activator Tert-butyl hydroquinone (TBHQ). Meanwhile, the protein expression levels of Keap1 was increased, and heat shock proteins (HSP70, HSP90 and HSPB1) were reduced after DCG066 treatment. In conclusion, this study confirmed that DCG066 inhibits MM proliferation and induces ferroptosis via the Nrf2/HO-1 pathway.








Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Kyle RA, Rajkumar SV (2008) Multiple myeloma. Blood 111(6):2962–2972. https://doi.org/10.1182/blood-2007-10-078022
Huang J, Chan SC, Lok V, Zhang L, Lucero-Prisno DE, Xu W, Zheng Z-J, Elcarte E, Withers M, Wong MCS (2022) The epidemiological landscape of multiple myeloma: a global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol 9(9):e670–e677. https://doi.org/10.1016/s2352-3026(22)00165-x
Liu J, Liu W, Mi L, Zeng X, Cai C, Ma J, Wang L (2019) Incidence and mortality of multiple myeloma in China, 2006–2016: an analysis of the global burden of Disease Study 2016. J Hematol Oncol 12(1). https://doi.org/10.1186/s13045-019-0807-5
Dimopoulos MA, Merlini G, Bridoux F, Leung N, Mikhael J, Harrison SJ, Kastritis E, Garderet L, Gozzetti A, van de Donk NWCJ, Weisel KC, Badros AZ, Beksac M, Hillengass J, Mohty M, Ho PJ, Ntanasis-Stathopoulos I, Mateos M-V, Richardson P, Blade J, Moreau P, San-Miguel J, Munshi N, Rajkumar SV, Durie BGM, Ludwig H, Terpos E (2023) Management of multiple myeloma-related renal impairment: recommendations from the International Myeloma Working Group. Lancet Oncol 24(7):e293–e311. https://doi.org/10.1016/s1470-2045(23)00223-1
De Luca F, Allegra A, Di Chio C, Previti S, Zappalà M, Ettari R (2023) Monoclonal antibodies: the Greatest Resource to treat multiple myeloma. Int J Mol Sci 24(4). https://doi.org/10.3390/ijms24043136
Minnie SA, Hill GR (2020) Immunotherapy of multiple myeloma. J Clin Invest 130(4):1565–1575. https://doi.org/10.1172/jci129205
Mou Y, Wang J, Wu J, He D, Zhang C, Duan C, Li B (2019) Ferroptosis, a new form of cell death: opportunities and challenges in cancer. J Hematol Oncol 12(1). https://doi.org/10.1186/s13045-019-0720-y
Rochette L, Dogon G, Rigal E, Zeller M, Cottin Y, Vergely C (2022) Lipid peroxidation and Iron metabolism: two Corner stones in the Homeostasis Control of Ferroptosis. Int J Mol Sci 24(1). https://doi.org/10.3390/ijms24010449
Zhang C, Liu X, Jin S, Chen Y, Guo R (2022) Ferroptosis in cancer therapy: a novel approach to reversing drug resistance. Mol Cancer 21(1). https://doi.org/10.1186/s12943-022-01530-y
Li F-J, Long H-Z, Zhou Z-W, Luo H-Y, Xu S-G, Gao L-C (2022) System Xc–/GSH/GPX4 axis: an important antioxidant system for the ferroptosis in drug-resistant solid tumor therapy. Front Pharmacol 13. https://doi.org/10.3389/fphar.2022.910292
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 152:175–185. https://doi.org/10.1016/j.freeradbiomed.2020.02.027
Casciello F, Windloch K, Gannon F, Lee JS (2015) Functional role of G9a histone methyltransferase in Cancer. Front Immunol 6. https://doi.org/10.3389/fimmu.2015.00487
San José-Enériz E, Agirre X, Rabal O, Vilas-Zornoza A, Sanchez-Arias JA, Miranda E, Ugarte A, Roa S, Paiva B, Estella-Hermoso de Mendoza A, Alvarez RM, Casares N, Segura V, Martín-Subero JI, Ogi F-X, Soule P, Santiveri CM, Campos-Olivas R, Castellano G, de Barrena MGF, Rodriguez-Madoz JR, García-Barchino MJ, Lasarte JJ, Avila MA, Martinez-Climent JA, Oyarzabal J, Prosper F (2017) Discovery of first-in-class reversible dual small molecule inhibitors against G9a and DNMTs in hematological malignancies. Nat Commun 8(1). https://doi.org/10.1038/ncomms15424
Zhang XY, Rajagopalan D, Chung T-H, Hooi L, Toh TB, Tian JS, Rashid MBMA, Sahib NRBM, Gu M, Lim JJ, Wang W, Chng WJ, Jha S, Chow EK-H (2020) Frequent upregulation of G9a promotes RelB-dependent proliferation and survival in multiple myeloma. Exp Hematol Oncol 9(1). https://doi.org/10.1186/s40164-020-00164-4
Segovia C, San José-Enériz E, Munera-Maravilla E, Martínez-Fernández M, Garate L, Miranda E, Vilas-Zornoza A, Lodewijk I, Rubio C, Segrelles C, Valcárcel LV, Rabal O, Casares N, Bernardini A, Suarez-Cabrera C, López-Calderón FF, Fortes P, Casado JA, Dueñas M, Villacampa F, Lasarte JJ, Guerrero-Ramos F, de Velasco G, Oyarzabal J, Castellano D, Agirre X, Prósper F, Paramio JM (2019) Inhibition of a G9a/DNMT network triggers immune-mediated bladder cancer regression. Nat Med 25(7):1073–1081. https://doi.org/10.1038/s41591-019-0499-y
Kondengaden SM, Luo L-f, Huang K, Zhu M, Zang L, Bataba E, Wang R, Luo C, Wang B, Li KK, Wang PG (2016) Discovery of novel small molecule inhibitors of lysine methyltransferase G9a and their mechanism in leukemia cell lines. Eur J Med Chem 122:382–393. https://doi.org/10.1016/j.ejmech.2016.06.028
Wang Y-f, Zhang J, Su Y, Shen Y-y, Jiang D-x, Hou Y-y, Geng M-y, Ding J, Chen Y (2017) G9a regulates breast cancer growth by modulating iron homeostasis through the repression of ferroxidase hephaestin. Nat Commun 8(1). https://doi.org/10.1038/s41467-017-00350-9
Torrente L, DeNicola GM (2022) Targeting NRF2 and its downstream processes: opportunities and challenges. Annu Rev Pharmacol Toxicol 62(1):279–300. https://doi.org/10.1146/annurev-pharmtox-052220-104025
Caprio C, Sacco A, Giustini V, Roccaro AM (2020) Epigenetic aberrations in multiple myeloma. Cancers 12(10). https://doi.org/10.3390/cancers12102996
Hogg SJ, Beavis PA, Dawson MA, Johnstone RW (2020) Targeting the epigenetic regulation of antitumour immunity. Nat Rev Drug Discov 19(11):776–800. https://doi.org/10.1038/s41573-020-0077-5
Bhat KP, Ümit Kaniskan H, Jin J, Gozani O (2021) Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat Rev Drug Discovery 20(4):265–286. https://doi.org/10.1038/s41573-020-00108-x
Prado-Romero DL, Medina-Franco JL (2021) Advances in the exploration of the epigenetic relevant chemical space. ACS Omega 6(35):22478–22486. https://doi.org/10.1021/acsomega.1c03389
Cao H, Li L, Yang D, Zeng L, Yewei X, Yu B, Liao G, Chen J (2019) Recent progress in histone methyltransferase (G9a) inhibitors as anticancer agents. Eur J Med Chem 179:537–546. https://doi.org/10.1016/j.ejmech.2019.06.072
Chen M-W, Hua K-T, Kao H-J, Chi C-C, Wei L-H, Johansson G, Shiah S-G, Chen P-S, Jeng Y-M, Cheng T-Y, Lai T-C, Chang J-S, Jan Y-H, Chien M-H, Yang C-J, Huang M-S, Hsiao M, Kuo M-L (2010) H3K9 histone methyltransferase G9a promotes lung cancer invasion and metastasis by silencing the cell adhesion molecule Ep-CAM. Cancer Res 70(20):7830–7840. https://doi.org/10.1158/0008-5472.Can-10-0833
Xu L, Gao X, Yang P, Sang W, Jiao J, Niu M, Liu M, Qin Y, Yan D, Song X, Sun C, Tian Y, Zhu F, Sun X, Zeng L, Li Z, Xu K (2021) EHMT2 inhibitor BIX-01294 induces endoplasmic reticulum stress mediated apoptosis and autophagy in diffuse large B-cell lymphoma cells. J Cancer 12(4):1011–1022. https://doi.org/10.7150/jca.48310
Dixon Scott J, Lemberg Kathryn M, Lamprecht Michael R, Skouta R, Zaitsev Eleina M, Gleason Caroline E, Patel Darpan N, Bauer Andras J, Cantley Alexandra M, Yang Wan S, Morrison B, Stockwell Brent R (2012) Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Jiang X, Stockwell BR, Conrad M (2021) Ferroptosis: mechanisms, biology and role in disease. Nat Rev Mol Cell Biol 22(4):266–282. https://doi.org/10.1038/s41580-020-00324-8
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci 113(34). https://doi.org/10.1073/pnas.1603244113
Hassannia B, Vandenabeele P, Vanden Berghe T (2019) Targeting ferroptosis to iron out cancer. Cancer Cell 35(6):830–849. https://doi.org/10.1016/j.ccell.2019.04.002
Yu L, Wang YF, Xiao J, Shen QQ, Chi SS, Gao YL, Lin DZ, Ding J, Fang YF, Chen Y (2023) Dysregulation of iron homeostasis by TfR-1 renders EZH2 wild type diffuse large B-cell lymphoma resistance to EZH2 inhibition. Acta Pharmacol Sin 44(10):2113–2124. https://doi.org/10.1038/s41401-023-01097-4
Dai Y, Hu L (2022) HSPB1 overexpression improves hypoxic-ischemic brain damage by attenuating ferroptosis in rats through promoting G6PD expression. J Neurophysiol 128(6):1507–1517. https://doi.org/10.1152/jn.00306.2022
Zhao Y, Li Y, Zhang R, Wang F, Wang T, Jiao Y (2020) The role of erastin in ferroptosis and its prospects in cancer therapy. OncoTargets Therapy 13:5429–5441. https://doi.org/10.2147/ott.S254995
Zhong Y, Tian F, Ma H, Wang H, Yang W, Liu Z, Liao A (2020) FTY720 induces ferroptosis and autophagy via PP2A/AMPK pathway in multiple myeloma cells. Life Sci 260. https://doi.org/10.1016/j.lfs.2020.118077
Ali A, Shafarin J, Unnikannan H, Al-Jabi N, Jabal RA, Bajbouj K, Muhammad JS, Hamad M (2021) Co-targeting BET bromodomain BRD4 and RAC1 suppresses growth, stemness and tumorigenesis by disrupting the c-MYC-G9a-FTH1axis and downregulation of HDAC1 in molecular subtypes of breast cancer. Int J Biol Sci 17(15):4474–4492. https://doi.org/10.7150/ijbs.62236
Dodson M, Castro-Portuguez R, Zhang DD (2019) NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol 23. https://doi.org/10.1016/j.redox.2019.101107
Kerins MJ, Ooi A (2018) The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal 29(17):1756–1773. https://doi.org/10.1089/ars.2017.7176
Hill KS, McDowell A, McCorkle JR, Schuler E, Ellingson SR, Plattner R, Kolesar JM (2021) KEAP1 is required for artesunate anticancer activity in non-small-cell lung cancer. Cancers 13(8). https://doi.org/10.3390/cancers13081885
Zhao J, Chen Y, Xiong T, Han S, Li C, He Y, He Y, Zhao G, Wang T, Wang L, Cheng T, Wang C, Wang J (2023) Clustered cobalt nanodots initiate ferroptosis by upregulating heme oxygenase 1 for radiotherapy sensitization. Small 19(10). https://doi.org/10.1002/smll.202206415
Jang JE, Eom J-I, Jeung H-K, Chung H, Kim YR, Kim JS, Cheong J-W, Min YH (2020) PERK/NRF2 and autophagy form a resistance mechanism against G9a inhibition in leukemia stem cells. J Exp Clin Cancer Res 39(1). https://doi.org/10.1186/s13046-020-01565-3
Jin T, Chen C (2022) Umbelliferone delays the progression of diabetic nephropathy by inhibiting ferroptosis through activation of the Nrf-2/HO-1 pathway. Food Chem Toxicol 163. https://doi.org/10.1016/j.fct.2022.112892
Yang J, Mo J, Dai J, Ye C, Cen W, Zheng X, Jiang L, Ye L (2021) Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis 12(11). https://doi.org/10.1038/s41419-021-04367-3
Xu Y, Li Y, Li J, Chen W (2022) Ethyl carbamate triggers ferroptosis in liver through inhibiting GSH synthesis and suppressing Nrf2 activation. Redox Biol 53. https://doi.org/10.1016/j.redox.2022.102349
Funding
This work was supported by the Natural Science Foundation of Shandong Province (number ZR2020QC083, ZR2021QH231, and ZR2022MH058) and Development Foundation of Xuzhou Medical University Affiliated Hospital (number XYFM202223).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study sonception. L.Z. and Y.Z.drafted the manuscript. L.W. and Z.W. designed the strategy. Y.Z. and X.W. and X.X. completed the biological experiments, X.L. and R.X. assisted in part of the in vitro experiments. All authors wrote, read, assisted in the revision, and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Y., Wang, X., Li, X. et al. Novel methyltransferase G9a inhibitor induces ferroptosis in multiple myeloma through Nrf2/HO-1 pathway. Ann Hematol (2024). https://doi.org/10.1007/s00277-024-05728-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-024-05728-6