Abstract
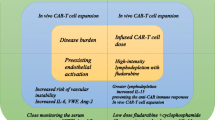
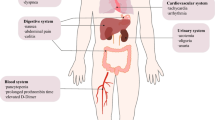
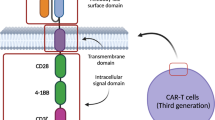
Chimeric antigen receptor T (CAR-T) cell therapy targeting CLL1 has been considered a potent weapon for patients with acute myeloid leukemia (AML). This study aims to evaluate the efficacy and toxicity of CLL1 CAR-T cell therapy in a larger cohort, with particular attention to cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Among the 32 patients assessed for efficacy, complete remission occurred in 71.88% (23/32) of cases and undetectable minimal residual disease in 14 patients. The CRS developed in all patients, with 8 individuals experiencing ICANS. Severe CRS and ICANS were observed in 11 and 2 patients, respectively. Furthermore, the Endothelial Activation and Stress Index (EASIX) and its derivatives measured before and after CLL1 CAR-T cell infusion were employed for predicting the severe complications. Significant differences were observed in EASIX scores on the day before lymphodepletion (Day BL, P = 0.023), −1 (P < 0.001), +1 (P < 0.001), and +3(P = 0.014); sEASIX scores on Day BL (P = 0.007), −1 (P < 0.001), +1 (P < 0.001), and +3 (P < 0.001); and mEASIX score on Day −1 (P = 0.004) between patients with mild and severe CRS/ICANS. Additionally, there was a significant difference in mEASIX scores between responders and non-responders on Day BL (P = 0.004) and Day −1 (P = 0.044). Our findings indicate that pre- and post-infusion assessments of EASIX/mEASIX/sEASIX scores serve as reliable prognostic indicators for severe CRS/ICANS and treatment response following CLL1 CAR-T cell therapy, which can assist physicians in implementing preemptive treatment strategies for potential severe complications and screening patients who are suitable candidates for CLL1 CAR-T cell therapy. EASIX/mEASIX/sEASIX scores serve as reliable prognostic indicators for severe CRS/ICANS following CLL1 CAR-T cell therapy. The preinfusion mEASIX scores of CLL1 CAR-T cells can effectively predict treatment response.





Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author at mingfengzhao@sina.com upon reasonable request.
References
O'Leary MC, Lu X, Huang Y, Lin X, Mahmood I, Przepiorka D, Gavin D, Lee S, Liu K, George B, Bryan W, Theoret MR, Pazdur R (2019) FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res : an official J Am Assoc Cancer Res 25(4):1142–1146. https://doi.org/10.1158/1078-0432.Ccr-18-2035
Locke FL, Miklos DB, Jacobson CA, Perales MA, Kersten MJ, Oluwole OO, Ghobadi A, Rapoport AP, McGuirk J, Pagel JM, Muñoz J, Farooq U, van Meerten T, Reagan PM, Sureda A, Flinn IW, Vandenberghe P, Song KW, Dickinson M et al (2022) Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. New England J Med 386(7):640–654. https://doi.org/10.1056/NEJMoa2116133
Marvin-Peek J, Savani BN, Olalekan OO, Dholaria B (2022) Challenges and advances in chimeric antigen receptor therapy for acute myeloid leukemia. Cancers 14(3). https://doi.org/10.3390/cancers14030497
Hofmann S, Schubert ML, Wang L, He B, Neuber B, Dreger P, Müller-Tidow C, Schmitt M (2019) Chimeric antigen receptor (CAR) T cell therapy in acute myeloid leukemia (AML). J Clin Med 8(2). https://doi.org/10.3390/jcm8020200
Marshall AS, Willment JA, Pyz E, Dennehy KM, Reid DM, Dri P, Gordon S, Wong SY, Brown GD (2006) Human MICL (CLEC12A) is differentially glycosylated and is down-regulated following cellular activation. Eur J Immunol 36(8):2159–2169. https://doi.org/10.1002/eji.200535628
van Rhenen A, van Dongen GA, Kelder A, Rombouts EJ, Feller N, Moshaver B, Stigter-van Walsum M, Zweegman S, Ossenkoppele GJ, Jan Schuurhuis G (2007) The novel AML stem cell associated antigen CLL-1 aids in discrimination between normal and leukemic stem cells. Blood 110(7):2659–2666. https://doi.org/10.1182/blood-2007-03-083048
Lu H, Zhou Q, Deshmukh V, Phull H, Ma J, Tardif V, Naik RR, Bouvard C, Zhang Y, Choi S, Lawson BR, Zhu S, Kim CH, Schultz PG (2014) Targeting human C-type lectin-like molecule-1 (CLL1) with a bispecific antibody for immunotherapy of acute myeloid leukemia. Angewandte Chemie (International ed in English) 53(37):9841–9845. https://doi.org/10.1002/anie.201405353
Jin X, Zhang M, Sun R, Lyu H, Xiao X, Zhang X, Li F, Xie D, Xiong X, Wang J, Lu W, Zhang H, Zhao M (2022) First-in-human phase I study of CLL-1 CAR-T cells in adults with relapsed/refractory acute myeloid leukemia. J Hematol Oncol 15(1):88. https://doi.org/10.1186/s13045-022-01308-1
Zhang H, Bu C, Peng Z, Li G, Zhou Z, Ding W, Zheng Y, He Y, Hu Z, Pei K, Luo M, Li C (2022) Characteristics of anti-CLL1 based CAR-T therapy for children with relapsed or refractory acute myeloid leukemia: the multi-center efficacy and safety interim analysis. Leukemia 36(11):2596–2604. https://doi.org/10.1038/s41375-022-01703-0
Ma YJ, Dai HP, Cui QY, Cui W, Zhu WJ, Qu CJ, Kang LQ, Zhu MQ, Zhu XM, Liu DD, Feng YF, Shen HJ, Liu TH, Qiu HY, Yu L, Wu DP, Tang XW (2022) Successful application of PD-1 knockdown CLL-1 CAR-T therapy in two AML patients with post-transplant relapse and failure of anti-CD38 CAR-T cell treatment. Am J Cancer Res 12(2):615–621
Schubert ML, Schmitt M, Wang L, Ramos CA, Jordan K, Müller-Tidow C, Dreger P (2021) Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol : official J Eur Soc Med Oncol 32(1):34–48. https://doi.org/10.1016/j.annonc.2020.10.478
Jacobson CA, Locke FL, Ma L, Asubonteng J, Hu ZH, Siddiqi T, Ahmed S, Ghobadi A, Miklos DB, Lin Y, Perales MA, Lunning MA, Herr MM, Hill BT, Ganguly S, Dong H, Nikiforow S, Hooper M, Kawashima J et al (2022) Real-world evidence of axicabtagene ciloleucel for the treatment of large B cell lymphoma in the United States. Transplant Cellul Ther 28(9):581.e581–581.e588. https://doi.org/10.1016/j.jtct.2022.05.026
Genoud V, Migliorini D (2023) Novel pathophysiological insights into CAR-T cell associated neurotoxicity. Front Neurol 14:1108297. https://doi.org/10.3389/fneur.2023.1108297
Luft T, Benner A, Jodele S, Dandoy CE, Storb R, Gooley T, Sandmaier BM, Becker N, Radujkovic A, Dreger P, Penack O (2017) EASIX in patients with acute graft-versus-host disease: a retrospective cohort analysis. Lancet Haematol 4(9):e414–e423. https://doi.org/10.1016/s2352-3026(17)30108-4
Luft T, Benner A, Terzer T, Jodele S, Dandoy CE, Storb R, Kordelas L, Beelen D, Gooley T, Sandmaier BM, Sorror M, Zeisbrich M, Radujkovic A, Dreger P, Penack O (2020) EASIX and mortality after allogeneic stem cell transplantation. Bone Marrow Transplant 55(3):553–561. https://doi.org/10.1038/s41409-019-0703-1
Korell F, Schreck N, Müller-Tidow C, Dreger P, Luft T (2022) Pre-transplant EASIX and sepsis after allogeneic stem cell transplantation. Intensive Care Med 48(6):753–755. https://doi.org/10.1007/s00134-022-06676-3
Varma A, Rondon G, Srour SA, Chen J, Ledesma C, Champlin RE, Ciurea SO, Saliba RM (2020) Endothelial activation and stress index (EASIX) at admission predicts fluid overload in recipients of allogeneic stem cell transplantation. Biol Blood Marrow Transplant : J Am Soc Blood Marrow Transplant 26(5):1013–1020. https://doi.org/10.1016/j.bbmt.2020.01.028
Song GY, Jung SH, Kim K, Kim SJ, Yoon SE, Lee HS, Kim M, Ahn SY, Ahn JS, Yang DH, Kim HJ, Lee JJ (2020) Endothelial activation and stress index (EASIX) is a reliable predictor for overall survival in patients with multiple myeloma. BMC Cancer 20(1):803. https://doi.org/10.1186/s12885-020-07317-y
Park S, Go SI, Lee GW (2022) The Endothelial Activation and Stress Index (EASIX) score is an independent prognostic factor in patients with diffuse large B-cell lymphoma. BMC Cancer 22(1):816. https://doi.org/10.1186/s12885-022-09915-4
Merz A, Germing U, Kobbe G, Kaivers J, Jauch A, Radujkovic A, Hummel M, Benner A, Merz M, Dreger P, Luft T (2019) EASIX for prediction of survival in lower-risk myelodysplastic syndromes. Blood Cancer J 9(11):85. https://doi.org/10.1038/s41408-019-0247-z
Pennisi M, Sanchez-Escamilla M, Flynn JR, Shouval R, Alarcon Tomas A, Silverberg ML, Batlevi C, Brentjens RJ, Dahi PB, Devlin SM, Diamonte C, Giralt S, Halton EF, Jain T, Maloy M, Mead E, Palomba ML, Ruiz J, Santomasso B et al (2021) Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv 5(17):3397–3406. https://doi.org/10.1182/bloodadvances.2020003885
Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, Maus MV, Park JH, Mead E, Pavletic S, Go WY, Eldjerou L, Gardner RA, Frey N, Curran KJ, Peggs K, Pasquini M, DiPersio JF, van den Brink MRM et al (2019) ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant : J Am Soc Blood Marrow Transplant 25(4):625–638. https://doi.org/10.1016/j.bbmt.2018.12.758
Arber DA, Orazi A, Hasserjian RP, Borowitz MJ, Calvo KR, Kvasnicka HM, Wang SA, Bagg A, Barbui T, Branford S, Bueso-Ramos CE, Cortes JE, Dal Cin P, DiNardo CD, Dombret H, Duncavage EJ, Ebert BL, Estey EH, Facchetti F et al (2022) International consensus classification of myeloid neoplasms and acute leukemias: integrating morphologic, clinical, and genomic data. Blood 140(11):1200–1228. https://doi.org/10.1182/blood.2022015850
Schorr C, Perna F (2022) Targets for chimeric antigen receptor T-cell therapy of acute myeloid leukemia. Front Immunol 13:1085978. https://doi.org/10.3389/fimmu.2022.1085978
Morsink LM, Walter RB, Ossenkoppele GJ (2019) Prognostic and therapeutic role of CLEC12A in acute myeloid leukemia. Blood Rev 34:26–33. https://doi.org/10.1016/j.blre.2018.10.003
Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, Wang W, Xu L, Liao S, Liu W, Wang Y, Liu N, Zhang J, Xia X, Kang T, Chen G, Cai X, Yang H, Zhang X et al (2018) CAR-T cells targeting CLL-1 as an approach to treat acute myeloid leukemia. J Hematol Oncol 11(1):7. https://doi.org/10.1186/s13045-017-0553-5
Liu Y, Fang Y, Chen X, Wang Z, Liang X, Zhang T, Liu M, Zhou N, Lv J, Tang K, Xie J, Gao Y, Cheng F, Zhou Y, Zhang Z, Hu Y, Zhang X, Gao Q, Zhang Y, Huang B (2020) Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sc Immunol 5(43). https://doi.org/10.1126/sciimmunol.aax7969
Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, Traversari C, Bordignon C, Ciceri F, Ostuni R, Bonini C, Casucci M, Bondanza A (2018) Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med 24(6):739–748. https://doi.org/10.1038/s41591-018-0036-4
Gust J, Hay KA, Hanafi LA, Li D, Myerson D, Gonzalez-Cuyar LF, Yeung C, Liles WC, Wurfel M, Lopez JA, Chen J, Chung D, Harju-Baker S, Özpolat T, Fink KR, Riddell SR, Maloney DG, Turtle CJ (2017) Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov 7(12):1404–1419. https://doi.org/10.1158/2159-8290.Cd-17-0698
Santomasso BD, Park JH, Salloum D, Riviere I, Flynn J, Mead E, Halton E, Wang X, Senechal B, Purdon T, Cross JR, Liu H, Vachha B, Chen X, DeAngelis LM, Li D, Bernal Y, Gonen M, Wendel HG et al (2018) Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov 8(8):958–971. https://doi.org/10.1158/2159-8290.Cd-17-1319
Korell F, Penack O, Mattie M, Schreck N, Benner A, Krzykalla J, Wang Z, Schmitt M, Bullinger L, Müller-Tidow C, Dreger P, Luft T (2022) EASIX and severe endothelial complications after CD19-directed CAR-T cell therapy-a cohort study. Front Immunol 13:877477. https://doi.org/10.3389/fimmu.2022.877477
Greenbaum U, Strati P, Saliba RM, Torres J, Rondon G, Nieto Y, Hosing C, Srour SA, Westin J, Fayad LE, Lee HJ, Iyer SP, Nair R, Nastoupil LJ, Parmar S, Rodriguez MA, Samaniego F, Steiner RE, Wang M et al (2021) CRP and ferritin in addition to the EASIX score predict CAR-T-related toxicity. Blood Adv 5(14):2799–2806. https://doi.org/10.1182/bloodadvances.2021004575
Acknowledgements
The authors thank the patients and their families for contributing to this study.
Funding
This work was supported by grants from the General Project of the National Natural Science Foundation of China (81970180 to MFZ), the Science and Technology Project of Tianjin Municipal Health Committee (TJWJ2022QN030 to MFZ), Key Projects of Tianjin Applied Basic Research and Multi-Investment Fund (21JCZDJC01240), Science and Technology Project of Tianjin Municipal Health Committee (TJWJ2022XK018 to MFZ), and the Key Science and Technology Support Project of Tianjin Science and Technology Bureau (20YFZCSY00800 to MFZ), as well as Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK-056B).
Author information
Authors and Affiliations
Contributions
Conceptualization of the project: YFZ, XMZ, MZ, XX, and MFZ.
Development of methodology: YFZ.
Manufacturing of CLL1 CAR-T products: XMZ and MZ.
Management of patients: RTG, YZ, YDP, HBZ, PJL, YZ, XYH, CCL, and HRL.
Collection, analysis, and interpretation of data: RTG, YZ, YDP, HBZ, PJL, YZ, XYH, CCL, and HRL.
Writing articles and revision of the manuscript: YFZ, XX, and MFZ.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Ethics approval
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin First Center Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 7722 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Zhang, X., Zhang, M. et al. Modified EASIX scores predict severe CRS/ICANS in patients with acute myeloid leukemia following CLL1 CAR-T cell therapy. Ann Hematol 103, 969–980 (2024). https://doi.org/10.1007/s00277-024-05617-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-024-05617-y