Abstract
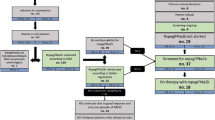
In patients with low-risk polycythemia vera, exposure to low-dose Ropeginterferon alfa-2b (Ropeg) 100 µg every 2 weeks for 2 years was more effective than the standard treatment of therapeutic phlebotomy in maintaining target hematocrit (HCT) (< 45%) with a reduction in the need for phlebotomy without disease progression. In the present paper, we analyzed drug survival, defined as a surrogate measure of the efficacy, safety, adherence, and tolerability of Ropeg in patients followed up to 5 years. During the first 2 years, Ropeg and phlebotomy-only (Phl-O) were discontinued in 33% and 70% of patients, respectively, for lack of response (12 in the Ropeg arm vs. 34 in the Phl-O arm) or adverse events (6 vs. 0) and withdrawal of consent in (3 vs. 10). Thirty-six Ropeg responders continued the drug for up to 3 years, and the probability of drug survival after a median of 3.15 years was 59%. Notably, the primary composite endpoint was maintained in 97%, 94%, and 94% of patients still on drug at 3, 4, and 5 years, respectively, and 60% of cases were phlebotomy-free. Twenty-three of 63 Phl-O patients (37%) failed the primary endpoint and were crossed over to Ropeg; among the risk factors for this failure, the need for more than three bloodletting procedures in the first 6 months emerged as the most important determinant. In conclusion, to improve the effectiveness of Ropeg, we suggest increasing the dose and using it earlier driven by high phlebotomy need in the first 6 months post-diagnosis.


Similar content being viewed by others
Data availability
No shared data are available.
References
Gerds AT, Gotlib J, Ali H, Bose P, Dunbar A, Elshoury A et al (2022) Myeloproliferative neoplasms, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 20(9):1033–1062
Tefferi A, Barbui T (2023) Polycythemia vera: 2024 update on diagnosis, risk-stratification, and management. Am J Hematol 98(9):1465–1487
Gisslinger H, Zagrijtschuk O, Buxhofer-Ausch V, Thaler J, Schloegl E, Gastl GA et al (2015) Ropeginterferon alfa-2b, a novel IFNalpha-2b, induces high response rates with low toxicity in patients with polycythemia vera. Blood 126(15):1762–1769
Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA et al (2009) IFNa activates dormant haematopoietic stem cells in vivo. Nature 458(7240):904–908
Mullally A, Bruedigam C, Poveromo L, Heidel FH, Purdon A, Vu T et al (2013) Depletion of Jak2V617F myeloproliferative neoplasm-propagating stem cells by interferon-a in a murine model of polycythemia vera. Blood 121(18):3692–3702
Gisslinger H, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyedet M et al (2020) Ropeginterferon alfa-2b versus standard therapy for polycythaemia vera (PROUD-PV and CONTINUATION-PV): a randomised, non-inferiority, phase 3 trial and its extension study. Lancet Haematol 7(3):e196-208
Kiladjian JJ, Chomienne C, Fenaux P (2008) Interferon-alpha therapy in bcr-abl-negative myeloproliferative neoplasms. Leukemia 22(11):1990–1998
Kiladjian JJ, Mesa RA, Hoffman R (2011) The renaissance of interferon therapy for the treatment of myeloid malignancies. Blood 117(18):4706–4715
Kiladjian JJ, Klade C, Georgiev P, Krochmalczyk D, Gercheva-Kyuchukova L, Egyedet M et al (2022) Long-term outcomes of polycythemia vera patients treated with ropeginterferon Alfa-2b. Leukemia 36(5):1408–1411
Barbui T, Vannucchi AM, De Stefano V, Masciulli A, Carobbio A, Ferrari A et al (2021) Ropeginterferon alfa-2b versus phlebotomy in low-risk patients with polycythaemia vera (Low-PV study): a multicentre, randomised phase 2 trial. Lancet Haematol 8(3):e175–e184
Barbui T, Vannucchi AM, De Stefano V, Carobbio A, Ghirardi A, Carioli G et al (2023) Ropeginterferon versus standard therapy for low-risk patients with polycythemia vera. NEJM Evid 2:6. https://doi.org/10.1056/EVIDoa2200335
Yiu ZZN, Becher G, Kirby B, Laws P, Reynolds NJ, Smith CH et al (2022) Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol 158(10):1131–1141
Funding
Low-PV is sponsored by FROM, Fondazione per la Ricerca Ospedale di Bergamo ETS and endorsed by the AIRC–Gruppo Italiano Malattie Mieloproliferative (AGIMM) with the program number 1005 and MYNERVA project with program number 21267, website at https://progettomynerva.it. Drug supply (Ropeginterferon alfa-2b) and financial support were provided by AOP Health (Vienna, Austria).
Author information
Authors and Affiliations
Contributions
TB conceived and designed the study, supervised the analysis, and wrote the paper. AC performed statistical analysis and contributed to manuscript writing. FF was in charge of project and data management. AG and GC critically revised the data analysis. AMV revised the study and contributed to manuscript writing. LC and FG were in charge of the management of samples for the biological subproject. AAL collected and analyzed data for the validation set from the Spanish registry. VDS, ERo, FC, MBo, AI, FPal, GB, FPan, ARi, GC, MC, DR, CM, SS, ERu, AP, NC, BM, EC, GGL, PG, SB, FR, FL, LS, CB, DC, NV, MBe, MCF, GT, and ARa collected data. All authors revised and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Barbui, T., Carobbio, A., De Stefano, V. et al. Ropeginterferon phase 2 randomized study in low-risk polycythemia vera: 5-year drug survival and efficacy outcomes. Ann Hematol 103, 437–442 (2024). https://doi.org/10.1007/s00277-023-05577-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05577-9