Abstract
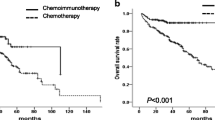
The current chemotherapy treatments have led to an improvement in survival rates for pediatric Burkitt’s lymphoma (BL). Survival in children with high-grade, mature B-cell non-Hodgkin’s lymphoma (B-NHL) has been prolonged by six rituximab doses combined with chemotherapy, whereas the efficacy of four doses has not been reported. This study aimed to explore optimal therapeutic strategies—the number of doses of rituximab based on different risk groups—and also aim to investigate the clinical characteristics of Chinese pediatric BL. This study consecutively enrolled children with BL in Beijing Children’s Hospital who received French-American-British mature B-cell lymphoma 96 (FAB/LMB96). The patients were divided into three groups: R0 group (chemotherapy alone), R6 group (chemotherapy combined with six rituximab doses), and R4 group (chemotherapy combined with four rituximab doses). The clinical characteristics and outcomes were evaluated. Univariate and multivariate analyses and prognostic nomogram were used to assess prognostic factors. A nomogram was developed that predicted overall survival based on the Cox proportional hazards model, and the concordance index (C-index) and a calibration curve were used to determine its predictive and discriminatory capacity. We enrolled 385 boys and 71 girls, with a median age of 6 years (1–14 years). Of these, 296 patients (65%) had initial abdominal symptoms, 182 (40%) had bulky disease, 46 (10%) had B symptoms, 77 (16.9%) had BL-ALL (blasts ≥ 25% in bone marrow (BM)), 96 (21%) had central nervous system (CNS) disease, 406 (89%) were in stages III–IV, 378 (83%) were in group C, 170 (37.2%) had lactate dehydrogenase (LDH) levels ≥ 1000 U/L at initial diagnosis, and 137 (30%) had tumor lysis syndrome. The R0, R6, and R4 groups included 79, 144, and 227 patients, respectively. Six patients were excluded due to treatment withdrawal for various reasons. The 3-year overall survival (OS) and event-free survival (EFS) percentages were 92% ± 1.3% and 91.3% ± 1.3%, respectively, in all cohorts, whereas the 3-year EFS percentage was 83.5% ± 4.2%, 93% ± 2.1%, and 92.9% ± 1.8% in the R0, R6, and R4 groups, respectively (P = 0.025). The nomogram included four important variables based on a multivariate analysis of the primary cohort: course of disease ≤ 20 days, presence of bulky disease at the beginning of diagnosis, central nervous system(CNS) invasion, and dosage of rituximab. The calibration curve showed that the nomogram was able to predict 3-year OS accurately. The C-index of the nomogram for OS prediction was 0.79 for both cohorts. In our hospital, pediatric BL was more commonly observed in school-age boys with an abdominal mass and mostly in advanced stages at initial diagnosis. The FAB/LMB96 regimen combined with rituximab significantly increased survival outcomes. We observed no significant differences between four and six doses of rituximab in terms of treatment outcomes. The proposed nomogram provides an individualized risk estimate of OS in patients with BL and may assist treatment decision-making or rituximab dose design.










Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in the article or supplementary information files. Raw datasets are available from the corresponding authors on reasonable request.
Abbreviations
- R:
-
Rituximab
- CT:
-
Chemotherapy
- BL:
-
Burkitt’s lymphoma
- BCH:
-
Beijing Children’s Hospital
- FISH:
-
Fluorescence in situ hybridization
- NHL:
-
Non-Hodgkin’s lymphoma
- CNS:
-
Central nervous system
- BM:
-
Bone marrow
- OS:
-
Overall survival
- CR:
-
Complete remission
- PR:
-
Partial remission
- SD:
-
Stable disease
- PD:
-
Progress disease
- R:
-
Recurrence
- LMB:
-
Lymphomes malins B
- WHO:
-
World Health Organization
- EFS:
-
Event-free survival
References
Patte C, Philip T, Rodary C, Zucker JM, Gerrard M, Behrendt H et al (1991) High survival rate in advanced stage B-cell lymphomas and leukemias without CNS involvement with a short intensive poly chemotherapy: results from the French Pediatric Oncology Society of a randomized trial of 216 children. J Clin Oncol 9:123–132. https://doi.org/10.1200/JCO.1991.9.1.123
Patte C, Auperin A, Michon J, Behrendt H, Leverger G, Frappaz D et al (2001) The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood 97:3370–3379. https://doi.org/10.1182/blood.v97.11.3370
Cairo MS, Gerrard M, Sposto R, Auperin A, Pinkerton CR, Michon J (2006) Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukaemia in children and adolescents. Blood 109:2736–2743. https://doi.org/10.1182/blood-2006-07-036665
Minard CV, Auperin A, Pillon M, Burke A, Anderson JR, Donald A et al (2016) Results of the randomized intergroup trial Inter-B-NHL Ritux 2010 for children and adolescents with high-risk B-cell non-Hodgkin lymphoma (B-NHL) and mature acute leukemia (B-AL): evaluation of Rituximab (R) efficacy in addition to standard LMB chemotherapy (CT) regimen. J Clin Oncol 34:10507. https://doi.org/10.1200/JCO.2016.34.15_suppl.10507
Jourdain A, Auperin A, Minard-Colin V, Aladjidi N, Zsiros J, Coze C (2015) Outcome of and prognostic factors for relapse in children and adolescents with mature B-cell lymphoma and leukemia treated in three consecutive prospective “Lymphomes Malins B” protocols. A Societe Francaise des Cancers de l’Enfant study. Haematologica 100:810–817. https://doi.org/10.3324/haematol.2014.121434
Cairo M, Auperin A, Perkins SL, Pinkerton R, Harrison L, Goldman S et al (2018) Overall survival of children and adolescents with mature B cell non-Hodgkin lymphoma who had refractory or relapsed disease during or after treatment with FAB/LMB 96: a report from the FAB/LMB 96 study group. Br J Haematol 182:859–869. https://doi.org/10.1111/bjh.15491
Zhang YH, Duan YL, Yang J, Jin L, Zhou CJ, Gao ZF (2008) Clinical study in 40 Burkitt and Burkitt-like pediatric lymphoma (in Chinese). Chin Pediatric J 46:209–214. https://doi.org/10.3321/j.issn:0578-1310.2008.03.012
Rosolen A, Perkins SL, Pinkerton CR et al (2015) Revised international pediatric non—Hodgkin lymphoma staging system. J Clin Oncol 33:2112–2118
Sandlund JT, Guillerman RP, Perkins SL et al (2015) International pediatric non-Hodgkin lymphoma response criteria. J Clin Oncol 33(18):2106–2111
Kimble Frazer J, Li KJ, Galardy PJ, Perkins SL, Auperin A, Cairo MS et al (2019) Excellent outcomes in children and adolescents with CNS+ Burkitt lymphoma or other mature B-NHL using only intrathecal and systemic chemo immunotherapy: results from FAB/LMB96 and COG ANHL01P1. Br J Haematol 185:374–377. https://doi.org/10.1111/bjh.15520
Woessmann W, Seidemann K, Mann G, Martin Z, Birgit B, Ilske O et al (2005) The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood 105:948–958. https://doi.org/10.1182/blood-2004-03-0973.17
Sun XF, Sun YS, Liu DG, Jiang WQ, He YQ, Lin TY et al (2004) Copmaring CHOP CHOP+ HD-MTX, and BFM-90 regimens in the survival rate of children and adolescents (in Chinese). Chin Cancer J 23:933–938. https://doi.org/10.3321/j.issn.1000-467X.2004.08.015
Minard-Colin V, Aupérin A, Pillon M (2020) Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med 382(23):2207–2219. https://doi.org/10.1056/NEJMoa1915315
Zhang M, Jing L, Yang J, Duan YL, Huang S, Zhang YH et al (2018) The clinical characteristics and treatment outcome of 186 pediatric Burkitt’s lymphoma (in Chinese). Chin Pediatric J 56:605–610. https://doi.org/10.3760/cma.j.issn.0578-1310.2018.08.010
Huang S, Jin L, Yang J, Duan YL, Zhang M, Zhang YH et al (2017) Clinical pathological characteristics and treatment outcome of 19 relapsed Burkitt’s lymphoma (in Chinese). Chin Pediatric J 55:748–753. https://doi.org/10.3760/cma.j.issn.0578-1310.2017.10.007
Shuang H, Ling J, Jing Y (2021) Treatment outcome in children with central nervous system-positive Burkitt lymphoma using only intrathecal and systemic chemotherapy combined with Rituximab: single-center experience from Beijing. Chin Med J (Engl). https://doi.org/10.1097/CM9.0000000000001386
Funding
This study was supported by the Special Fund of the Pediatric Medical Coordinated Development Center of Beijing Children’s Hospital (No. XTZD20180204), National Natural Science Foundation of China (NSFC) (No. 82070154), Beijing Natural Science Foundation (No. 7222056), and Capital’s Funds for Health Improvement and Research (CFH) (No. 2022-1-2091).
Author information
Authors and Affiliations
Contributions
SH, LJ, JY, YZ, and MZ collected and managed data. SH and YP analyzed the data. SH designed the research and wrote the paper. YD and HZ revised the paper. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Beijing Children’s Hospital (IEC-C008-A08-V.05.1).
Consent for publication
All patients signed informed consent sheets and consented to the publication of their anonymized data in this article.
Competing interests
The authors declare no competing interests.
Author highest academic degrees
Shuang Huang, Ling Jin, Meng Zhang, Yonghong Zhang, and Yanlong Duan have master’s degrees. Yaguang Peng and Huyong Zheng have doctor’s degrees.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, S., Jin, L., Yang, J. et al. Optimal dosage of rituximab for children with Burkitt lymphoma. Ann Hematol 103, 893–903 (2024). https://doi.org/10.1007/s00277-023-05568-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05568-w