Abstract
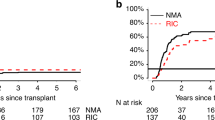
The current study includes all consecutive patients (N = 484) who received a reduced-intensity conditioning regimen (RIC) allogeneic hematopoietic stem cell transplantation in our center from 1999 to 2020. Conditioning regimens were based on fludarabine with melphalan or busulfan, with low-dose thiotepa and pharmacological GVHD prophylaxis consisted of cyclosporine A (CsA)-methotrexate (MTX)/mofetil (MMF) (n = 271), tacrolimus-sirolimus (n = 145), and post-transplantation cyclophosphamide (PTCy)-tacrolimus (n = 68). The median time of overall follow-up in survivors was 8 years (1–22 years) and was at least 3 years in all three GVHD prophylaxis groups. Thirty-three percent had a high or very high disease risk index, 56% ≥ 4 European bone marrow transplantation risk, and 65% ≥ 3 hematopoietic stem cell transplantation comorbidity index score-age score. Neutrophil and platelet engraftment was longer for PTCy-tacro (p 0.0001). Cumulative incidence of grade III–IV aGVHD was 17% at 200 days, and that of moderate-severe cGvHD was 36% at 8 years. GVHD prophylaxis was the only prognostic factor in the multivariable analyses for the development of aGVHD and moderate-severe cGVHD (p 0.0001). NRM and relapse incidences were 29% and 30% at 8 years, while OS and PFS rates were 43% and 39% at 8 years. At 3 years, OS was highest in the PTCy-tacro group (68%) than in the tacro-siro (61%) and CsA-MTX/MMF (49%) cohorts (p < 0.01). In the three groups, respectively, the 200-day incidence of grade III–IV aGvHD (6% vs. 12% vs. 23%) and 3-year moderate-severe cGVHD (8% vs. 40% vs. 38%) were lower in the PTCy cohort. These better outcomes were confirmed in multivariable analyses. Based on our recent results, the PTCy could be considered as a real GvHD prophylaxis in the RIC setting due to improve best 3-year GvHD and survival outcomes.





Similar content being viewed by others
References
Copelan EA (2006) Hematopoietic stem-cell transplantation. N Engl J Med 354:1813–1826
Spyridonidis A, Labopin M, Savani BN et al (2020) Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transplant 55(6):1114–1125
Horowitz MM, Gale RP, Sondel PM et al (1990) Graft-versus-leukemia reactions after bone marrow transplantation. Blood 75(555):562
Nakamura R, Forman SJ (2014) Reduced intensity conditioning for allogeneic hematopoietic cell transplantation: considerations for evidence-based GVHD prophylaxis. Expert Rev Hematol 7(3):407–421
Giralt S, Thall PF, Khouri I et al (2001) Melphalan and purine analog-containing preparative regimens: reduced-intensity conditioning for patients with hematologic malignancies undergoing allogeneic progenitor cell transplantation. Blood 97:631–637
Slavin S, Nagler A, Naparstek E et al (1998) Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood 91:756–763
McSweeney PA, Niederwieser D, Shizuru JA et al (2001) Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing highdose cytotoxic therapy with graft-versus-tumor effects. Blood 97:3390–3400
Childs R, Chernoff A, Contentin N et al (2000) Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med 343:750–758
Baron F, Labopin M, Peniket A et al (2015) Reduced-intensity conditioning with fludarabine and busulfan versus fludarabine and melphalan for patients with acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European Group for Blood and Marrow Transplantation. Cancer 121:1048–1055
Damlaj M, Alkhateeb HB, Hefazi M et al (2016) Fludarabine-busulfan reduced-intensity conditioning in comparison with fludarabine-melphalan is associated with increased relapse risk in spite of pharmacokinetic dosing. Biol Blood Marrow Transplant 22(8):1431–1439
Shimoni A, Hardan I (2007) Shem-Tov N et al Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia 21(10):2109–2116
Jain T, Alahdab F, Firwana B, Sonbol MB, Almader-Douglas D, Palmer J (2019) Choosing a reduced-intensity conditioning regimen for allogeneic stem cell transplantation, fludarabine/busulfan versus fludarabine melphalan: a systematic review and meta-analysis. Biol Blood Marrow Transplant 25(4):728–733
Zhou Z, Nath R, Cerny J et al (2020) Reduced intensity conditioning for acute myeloid leukemia using melphalan- vs busulfan-based regimens: a CIBMTR report. Blood Adv 4(13):3180–3190
Storb R, Deeg HJ, Whitehead J et al (1986) Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314(12):729–735
Ratanatharathorn V, Nash RA, Przepiorka D et al (1998) Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 92(7):2303–2314
Nash RA, Antin JH, Karanes C et al (2000) Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 96(6):2062–2068
Khouri IF, Keating M, Korbling M et al (1998) Transplant-lite: induction of graft-versus-malignancy using fludarabine-based nonablative chemotherapy and allogeneic blood progenitor-cell transplantation as treatment for lymphoid malignancies. J Clin Oncol 16(8):2817–2824
Oran B, Giralt S, Saliba R et al (2007) Allogeneic hematopoietic stem cell transplantation for the treatment of high-risk acute myelogenous leukemia and myelodysplastic syndrome using reduced-intensity conditioning with fludarabine and melphalan. Biol Blood Marrow Transplant 13(4):454–462
Pérez-Simón JA, Martino R, Caballero D et al (2008) Reduced-intensity conditioning allogeneic transplantation from unrelated donors: evaluation of mycophenolate mofetil plus cyclosporin A as graft-versus-host disease prophylaxis. Biol Blood Marrow Transplant 14(6):664–671
Mielcarek M, Martin PJ, Leisenring W et al (2003) Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood 102(2):756–762
Neumann F, Graef T, Tapprich C et al (2005) Cyclosporine A and mycophenolate mofetil vs cyclosporine A and methotrexate for graft-versus-host disease prophylaxis after stem cell transplantation from HLA-identical siblings. Bone Marrow Transplant 35(11):1089–1093
Chhabra S, Liu Y, Hemmer TM et al (2019) Comparative analysis of calcineurin-inhibitor-based methotrexate and mycophenolate mofetil-containing regimens for prevention of graft-versus-host disease after reduced intensity conditioning allogeneic transplantation. Biol Blood Marrow Transplant 25(1):73–85
Cutler C, Kim HT, Hochberg E et al (2004) Sirolimus and tacrolimus without methotrexate as graft-versus-host disease prophylaxis after matched related donor peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 10(5):328–336
Cutler C, Li S, Ho VT et al (2007) Extended follow-up of methotrexate-free immunosuppression using sirolimus and tacrolimus in related and unrelated donor peripheral blood stem cell transplantation. Blood 109(7):3108–14. 6
Kharfan-Dabaja MA, Parody R, Perkins J et al (2017) Tacrolimus plus sirolimus with or without ATG as GVHD prophylaxis in HLA-mismatched unrelated donor allogeneic stem cell transplantation. Bone Marrow Transplant 52(3):438–444
Rodriguez R, Nakamura R, Palmer JM et al (2010) A phase II pilot study of tacrolimus/sirolimus GVHD prophylaxis for sibling donor hematopoietic stem cell transplantation using 3 conditioning regimens. Blood 115(5):1098–1105
Perez-Simón JA, Martino R, Parody R et al (2013) The combination of sirolimus plus tacrolimus improves outcome after reduced-intensity conditioning, unrelated donor hematopoietic stem cell transplantation compared with cyclosporine plus mycofenolate. Haematologica 98(4):526–532
Parody R, López-Corral L, Lopez-Godino O et al (2016) GvHD prophylaxis with tacrolimus plus sirolimus after reduced intensity conditioning allogeneic transplantation: results of a multicenter study. Bone Marrow Transplant 51(11):1524–1526
Snyder DS, Palmer J, Gaal K et al (2010) Improved outcomes using tacrolimus/sirolimus for graft-versus-host disease prophylaxis with a reduced-intensity conditioning regimen for allogeneic hematopoietic cell transplant as treatment of myelofibrosis. Biol Blood Marrow Transplant 16(2):281–286
Nakamura R, Palmer JM, O’Donnell MR et al (2012) Reduced intensity allogeneic hematopoietic stem cell transplantation for MDS using tacrolimus/sirolimus-based GVHD prophylaxis. Leuk Res 36(9):1152–1156
Martino R, Caballero MD, Canals C et al (2001) Allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning: results of a prospective multicentre study. Br J Haematol 115(3):653–659
García-Cadenas I, Awol R, Esquirol AS et al (2020) Incorporating posttransplant cyclophosphamide-based prophylaxis as standard-of-care outside the haploidentical setting: challenges and review of the literature. Bone Marrow Transplant 55(6):1041–1049
O’Donnell PV, Luznik L, Jones RJ et al (2002) Nonmyeloablative bone marrow transplantation from partially HLA-mismatched related donors using posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14:377–386
Luznik L, O’Donnell PV, Symons HJ et al (2008) HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14:641–650
Esquirol A, Pascual MJ (2017) M Ortiz et al Single-agent GvHD prophylaxis with tacrolimus after post-transplant high-dose cyclophosphamide is a valid option for haploidentical transplantation in adults with hematological malignancies. Bone Marrow Transplant 52(9):1273–1279
Sorror ML, Storb RF, Sandmaier BM et al (2014) Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol 32:3249–3256
Gratwohl A (2012) The EBMT risk score. BoneMarrow Transplant 47:749–756
Armand P, Kim HT, Logan BR et al (2014) Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 123:3664–3671
Ruggeri A, Labopin M (2021) Angelucci E et al Prognostic factors for neutrophil engraftment after haploidentical cell transplantation with PT-Cy in patients with acute myeloid leukemia in complete remission, on behalf of the ALWP-EBMT. Bone Marrow Transplant 56(8):1842–1849
Shimoni A, Labopin M (2016) Savani B et al Long-term survival and late events after allogeneic stem cell transplantation from HLA-matched siblings for acute myeloid leukemia with myeloablative compared to reduced-intensity conditioning: a report on behalf of the acute leukemia working party of European group for blood and marrow transplantation. J Hematol Oncol 9(1):118
Martino R, Henseler A, van Lint M et al (2017) Long-term follow-up of a retrospective comparison of reduced-intensity conditioning and conventional high-dose conditioning for allogeneic transplantation from matched related donors in myelodysplastic syndromes. Bone Marrow Transplant 52(8):1107–1112
Scott BL, Pasquini MC (2021) Fei M et al Myeloablative versus reduced-intensity conditioning for hematopoietic cell transplantation in acute myelogenous leukemia and myelodysplastic syndromes—long-term follow-up of the BMT CTN 0901 Clinical Trial. Transplant Cell Ther 27(6):483.e1-483.e6
Bolaños-Meade J, Hamadani M, Wu J et al (2023) Post-transplantation cyclophosphamide-based graft-versus-host disease prophylaxis. N Engl J Med 388(25):2338–2348
Wachsmuth LP, Patterson MT, Eckhaus MA et al (2019) Posttransplantation cyclophosphamide prevents graft-versus host disease by inducing alloreactive T cell dysfunction and suppression. J Clin Invest 129(6):2357–2373
Esquirol A, Pascual MJ, Garcia-Cadenas I et al (2021) Combining three different pretransplantation scores improves predictive value in patients after haploidentical stem cell transplantation with thiotepa, busulfan, and fludarabine conditioning and post-transplantation cyclophosphamide. Transplant Cell Ther 27(7):614.e1-6148.e1
Tichelli A, Beohou E (2019) Labopin M et al Evaluation of second solid cancers after hematopoietic stem cell transplantation in European patients. JAMA Oncol 5(2):229–235
Funding
This project was funded by “La Marató de TV3” Foundation 2019 41–30, AGAUR 2017 SGR 1395, and 2021 SGR 01139 from the Catalan Government and a grant from “Obra Social La Caixa,” Barcelona, Spain. PERIS SLT002/16/0043 from the Catalan Government and FIS PI17/01246, PI20/01621, and RD16/0011/0028 from the Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Spain.
Author information
Authors and Affiliations
Contributions
A.E. designed the study, performed research, analyzed data, and wrote the manuscript; R.M. and J.S. designed the study and performed research; I.G.C., S.N, A.G, A.C.C., G.O., J.L., S.R., M.A., S.S., C.M., and J.B. contributed essential data; and all authors approved the final version of the manuscript. R.M. and J.S. contributed equally to this work as senior consultant.
Corresponding author
Ethics declarations
Declarations
No human were directly involved in the study; we analyzed the final long-term outcomes in the standard procedure (RIC-alloSCT), without testing a different strategy than clinical practices. Even so, all transplants were performed according to the approved institutional protocols, and all patients signed the informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jorge Sierra and Rodrigo Martino equally contributed as senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Esquirol, A., Cadenas, I.G., Novelli, S. et al. Outcome improvement over time in reduced intensity conditioning hematopoietic transplantation: a 20-year experience. Ann Hematol 103, 321–334 (2024). https://doi.org/10.1007/s00277-023-05530-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05530-w