Abstract
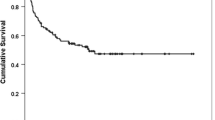
Autologous stem cell transplantation (ASCT) is a salvage therapy for relapsed or refractory diffuse large B-cell lymphoma (DLBCL). We have developed a novel conditioning regimen called CEAC (oral semustine 250 mg/m2 d-6, etoposide 300 mg/m2 d-5 ~ d-2, cytarabine 500 mg/m2 d-5 ~ d-2, and cyclophosphamide 1200 mg/m2 d-5 ~ d-2) In lymphoma patients in China. Here, we conducted a study to compare the conventional BEAM regimen with the CEAC regimen in 110 DLBCL patients. Propensity-score matching was performed in a 1:4 ratio (22 patients received BEAM and 88 received CEAC). Our results showed no significant difference in the overall response rate (95% vs 97%, P = 1.000) and complete response rate (66% vs 73%, P = 0.580) between the two cohorts. The 5-year progression-free survival (PFS), 5-year overall survival (OS), and 5-year cumulative incidence of relapse (CIR) for all patients were 72% (95% CI 62%–82%), 92% (95% CI 86%–97%), and 29% (95% CI 17%–38%), respectively. There was no significant difference in the 5-year PFS (80% vs 70%, P = 0.637), 5-year OS (95% vs 91%, P = 0.496), and 5-year CIR (20% vs 30%, P = 0.733) between cohorts. In terms of safety, the CEAC cohort had a lower incidence rate of grade 1–2 gastrointestinal hemorrhage (P = 0.023) and severe nausea (P = 0.007) compared with the BEAM cohort. In conclusion, the CEAC regimen seems to be a suitable alternative to the BEAM regimen for ASCT in DLBCL patients.


Similar content being viewed by others
Data availability
Data are available from the authors upon reasonable request.
References
Sehn LH, Salles G (2021) Diffuse Large B-Cell Lymphoma. N Engl J Med 384:842–858
Coiffier B, Thieblemont C, Van Den Neste E et al (2010) Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 116:2040–5
Crump M, Neelapu SS, Farooq U et al (2017) Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 130:1800–1808
Gisselbrecht C, Glass B, Mounier N et al (2010) Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 28:4184–4190
Colita A, Colita A, Bumbea H et al (2019) LEAM vs. BEAM vs. CLV Conditioning Regimen for Autologous Stem Cell Transplantation in Malignant Lymphomas. Retrospective Comparison of Toxicity and Efficacy on 222 Patients in the First 100 Days After Transplant, On Behalf of the Romanian Society for Bone Marrow Transplantation. Front Oncol 9:892
Caballero MD, Rubio V, Rifon J et al (1997) BEAM chemotherapy followed by autologous stem cell support in lymphoma patients: analysis of efficacy, toxicity and prognostic factors. Bone Marrow Transplant 20:451–458
Deveci B, Ateşoğlu EB, Bayrak E et al (2023) Comparative Efficacy and Safety of Beam and Team Conditioning Regimens for Autologous Stem Cell Transplantation in Lymphoma Patients. Transplant Proc 55:235–241
Costes-Tertrais D, Hueso T, Gastinne T et al (2022) Bendamustine-EAM versus R-BEAM after high-dose cytarabine-based induction in newly diagnosed patients with mantle cell lymphoma, a LYSA retrospective study. Bone Marrow Transplant 57:627–632
Robinson SP, Boumendil A, Finel H et al (2018) High-dose therapy with BEAC conditioning compared to BEAM conditioning prior to autologous stem cell transplantation for non-Hodgkin lymphoma: no differences in toxicity or outcome. A matched-control study of the EBMT-Lymphoma Working Party. Bone Marrow Transplantation. 53:1553–9
Geisler CH, Kolstad A, Laurell A et al (2012) Nordic MCL2 trial update: six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: still very long survival but late relapses do occur. Br J Haematol 158:355–362
Kuittinen T, Husso-Saastamoinen M, Sipola P et al (2005) Very acute cardiac toxicity during BEAC chemotherapy in non-Hodgkin’s lymphoma patients undergoing autologous stem cell transplantation. Bone Marrow Transplant 36:1077–1082
Kuittinen T, Jantunen E, Vanninen E et al (2006) Cardiac effects within 3 months of BEAC high-dose therapy in non-Hodgkin’s lymphoma patients undergoing autologous stem cell transplantation. Eur J Haematol 77:120–127
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32:3059–3068
Lin M, Wu X, Zhang L et al (2023) Fotemustine, etoposide, cytarabine, and cyclophosphamide (FEAC) conditioning regimen for autologous stem cell transplantation in lymphoma. Leuk Lymphoma 64:605–612
Kim KH, Lee JH, Lee M et al (2023) Busulfan, Melphalan, and Etoposide (BuME) Showed an Equivalent Effect to Busulfan, Cyclophosphamide, and Etoposide (BuCE) as Conditioning Therapy for Autologous Stem Cell Transplantation in Patients with Relapsed or High-Risk Non-Hodgkin’s Lymphoma: A Multicenter Randomized Phase II Study bythe Consortium for Improving Survival of Lymphoma (CISL). Cancer Res Treat 55:304–313
Cohen Y, Lebel E, Zimran E et al (2023) Long term results with thiotepa containing regimen (TECAM) for autologous stem cell transplantation. Transplant Cell Ther 29(8):505.e1–505.e8
Locke FL, Miklos DB, Jacobson CA et al (2022) Axicabtagene Ciloleucel as Second-Line Therapy for Large B-Cell Lymphoma. N Engl J Med 386:640–654
Kamdar M, Solomon SR, Arnason J et al (2022) Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet (London, England) 399:2294–2308
Bishop MR, Dickinson M, Purtill D et al (2022) Second-Line Tisagenlecleucel or Standard Care in Aggressive B-Cell Lymphoma. N Engl J Med 386:629–639
Neelapu SS, Jacobson CA, Ghobadi A et al (2023) Five-year follow-up of ZUMA-1 supports the curative potential of axicabtagene ciloleucel in refractory large B-cell lymphoma. Blood 141:2307–2315
Wang T, Xu L, Gao L et al (2023) Chimeric antigen receptor T-cell therapy combined with autologous stem cell transplantation improved progression-free survival of relapsed or refractory diffuse large B-cell lymphoma patients: A single-center, retrospective, cohort study. Hematol Oncol 10:891–900
Wang T, Gao L, Wang Y et al (2020) Hematopoietic stem cell transplantation and chimeric antigen receptor T cell for relapsed or refractory diffuse large B-cell lymphoma. Immunotherapy 12:997–1006
Acknowledgements
The authors thank the patients who have participated in the study. This work was supported by Science and Technology Commission of Shanghai Municipality (23YF1458800) and grants from the National Natural Science Foundation of China (NSFC) (82270202). We would like to thank MogoEdit (https://www.mogoedit.com) for its English editing during the preparation of this manuscript.
Funding
Tao Wang received funding from the Science and Technology Commission of Shanghai Municipality (23YF1458800), and Jianmin Yang received funding from the National Natural Science Foundation of China (NSFC) (82270202).
Author information
Authors and Affiliations
Contributions
JY, YL and GL designed the study approved the final version of the manuscript. TW and PL analyzed the data, wrote the manuscript. LX, LG, XN, GT, LC, JC, LW, YW, WF, WY, NL and RL collected the data and critically revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, T., Liu, P., Xu, L. et al. CEAC (oral semustine, etoposide, cytarabine and cyclophosphamide) vs BEAM (carmustine, etoposide, cytarabine, and melphalan) conditioning regimen of autologous stem cell transplantation for diffuse large B-cell lymphoma: a post-hoc, propensity score-matched, cohort study in Chinese patients. Ann Hematol 103, 575–582 (2024). https://doi.org/10.1007/s00277-023-05513-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05513-x