Abstract
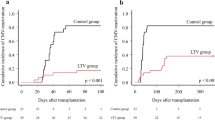
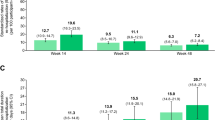
Cytomegalovirus (CMV) infection is a major infectious complication following allogeneic hematopoietic cell transplantation (allo-HCT). Although letermovir (LMV) prophylaxis dramatically reduces the incidence of early clinically significant CMV (csCMV) infection, it remains unclear whether it has a beneficial effect on nonrelapse mortality (NRM) and overall survival (OS). Herein, we evaluated the impact of LMV prophylaxis on posttransplant outcomes using the registry database of the Japanese Society for Transplantation and Cellular Therapy. Adult patients who underwent allo-HCT between 2017 and 2019 were analyzed (n = 6004). LMV prophylaxis was administered to 1640 patients (LMV group) and it significantly reduced the incidence of csCMV infection compared with those not administered LMV prophylaxis (15.4% vs 54.1%; p < 0.01). However, it did not improve the 1-year NRM (hazard ratio [HR], 0.93; p = 0.40) and OS (HR, 0.96; p = 0.49). In the LMV group, 74 patients had breakthrough csCMV infection and showed inferior NRM (HR, 3.44; p < 0.01) and OS (HR, 1.93; p = 0.02) compared with those without infection. After completing LMV prophylaxis, 252 patients had late csCMV infection and showed inferior NRM (HR, 1.83; p < 0.01) and OS (HR, 1.58; p < 0.01). Our findings suggest that managing breakthrough and late csCMV infections is important for improving long-term outcomes.





Similar content being viewed by others
Data availability
The data of this study is not publicly available due to ethical restrictions that it exceeds the scope of the recipient/donor’s consent for research use in the registry.
References
Boeckh M, Murphy WJ, Peggs KS (2015) Recent advances in cytomegalovirus: an update on pharmacologic and cellular therapies. Biol Blood Marrow Transplant 21(1):24–29. https://doi.org/10.1016/j.bbmt.2014.11.002
Boeckh M, Ljungman P (2009) How I treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 113(23):5711–5719
Ljungman P, Hakki M, Boeckh M (2010) Cytomegalovirus in hematopoietic stem cell transplant recipients. Infect Dis Clin N Am 24(2):319–337
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, Hubacek P, Navarro D, Cordonnier C, Ward KN European Conference on Infections in Leukaemia g (2019) Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19(8):e260–e272. https://doi.org/10.1016/S1473-3099(19)30107-0
Marty FM, Ljungman P, Papanicolaou GA, Winston DJ, Chemaly RF, Strasfeld L, Young JA, Rodriguez T, Maertens J, Schmitt M, Einsele H, Ferrant A, Lipton JH, Villano SA, Chen H, Boeckh M (2011) Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial. Lancet Infect Dis 11(4):284–292. https://doi.org/10.1016/S1473-3099(11)70024-X
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA, Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H et al (2017) Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 377(25):2433–2444. https://doi.org/10.1056/NEJMoa1706640
Green ML, Leisenring W, Xie H, Mast TC, Cui Y, Sandmaier BM, Sorror ML, Goyal S, Özkök S, Yi J, Sahoo F, Kimball LE, Jerome KR, Marks MA, Boeckh M (2016) Cytomegalovirus viral load and mortality after haemopoietic stem cell transplantation in the era of pre-emptive therapy: a retrospective cohort study. Lancet Haematol 3(3):e119–e127. https://doi.org/10.1016/s2352-3026(15)00289-6
Takenaka K, Nishida T, Asano-Mori Y, Oshima K, Ohashi K, Mori T, Kanamori H, Miyamura K, Kato C, Kobayashi N, Uchida N, Nakamae H, Ichinohe T, Morishima Y, Suzuki R, Yamaguchi T, Fukuda T (2015) Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation-related Complication Working Group. Biol Blood Marrow Transplant 21(11):2008–2016. https://doi.org/10.1016/j.bbmt.2015.07.019
Teira P, Battiwalla M, Ramanathan M, Barrett AJ, Ahn KW, Chen M, Green JS, Saad A, Antin JH, Savani BN, Lazarus HM, Seftel M, Saber W, Marks D, Aljurf M, Norkin M, Wingard JR, Lindemans CA, Boeckh M et al (2016) Early cytomegalovirus reactivation remains associated with increased transplant-related mortality in the current era: a CIBMTR analysis. Blood 127(20):2427–2438. https://doi.org/10.1182/blood-2015-11-679639
Hakki M, Aitken SL, Danziger-Isakov L, Michaels MG, Carpenter PA, Chemaly RF, Papanicolaou GA, Boeckh M, Marty FM (2021) American Society for Transplantation and Cellular Therapy Series: #3-prevention of cytomegalovirus infection and disease after hematopoietic cell transplantation. Transplant Cell Ther 27(9):707–719. https://doi.org/10.1016/j.jtct.2021.05.001
Yoshimura H, Satake A, Ishii Y, Ichikawa J, Saito R, Konishi A, Hotta M, Nakanishi T, Fujita S, Ito T, Ishii K, Nomura S (2022) Real-world efficacy of letermovir prophylaxis for cytomegalovirus infection after allogeneic hematopoietic stem cell transplantation: a single-center retrospective analysis. J Infect Chemother 28(9):1317–1323. https://doi.org/10.1016/j.jiac.2022.05.019
Liu LW, Yn A, Gao F, Olson M, Crain M, Abboud R, Westervelt P, Abboud C, Vij R, Stockerl-Goldstein K, Pusic I, Cashen AF, Schroeder MA (2022) Letermovir discontinuation at day 100 after allogeneic stem cell transplant is associated with increased CMV-related mortality. Transplant Cell Ther 28(8):510.e511–510.e519. https://doi.org/10.1016/j.jtct.2022.05.020
Freyer CW, Carulli A, Gier S, Ganetsky A, Timlin C, Schuster M, Babushok D, Frey NV, Gill SI, Hexner EO, Luger SM, Mangan JK, Martin ME, McCurdy SR, Perl AE, Porter DL, Pratz K, Smith J, Stadtmauer EA, Loren AW (2022) Letermovir vs. high-dose valacyclovir for cytomegalovirus prophylaxis following haploidentical or mismatched unrelated donor allogeneic hematopoietic cell transplantation receiving post-transplant cyclophosphamide. Leuk Lymphoma 63(8):1925–1933. https://doi.org/10.1080/10428194.2022.2042686
Malagola M, Turra A, Signorini L, Corbellini S, Polverelli N, Masina L, Del Fabro G, Lorenzotti S, Fumarola B, Farina M, Morello E, Radici V, Buttini EA, Colnaghi F, Bernardi S, Re F, Caruso A, Castelli F, Russo D (2022) Results of an innovative program for surveillance, prophylaxis, and treatment of infectious complications following allogeneic stem cell transplantation in hematological malignancies (BATMO Protocol). Front Oncol 12:874117. https://doi.org/10.3389/fonc.2022.874117
Akahoshi Y, Kimura SI, Tada Y, Matsukawa T, Tamaki M, Doki N, Uchida N, Tanaka M, Nakamae H, Kuriyama T, Matsuoka KI, Ikeda T, Kimura T, Fukuda T, Kanda Y, Atsuta Y, Murata M, Terakura S, Nakasone H (2022) Cytomegalovirus gastroenteritis in patients with acute graft-versus-host disease. Blood Adv 6(2):574–584. https://doi.org/10.1182/bloodadvances.2021005885
Wolfe D, Zhao Q, Siegel E, Puto M, Murphy D, Roddy J, Efebera Y, Tossey J (2021) Letermovir prophylaxis and cytomegalovirus reactivation in adult hematopoietic cell transplant recipients with and without acute graft versus host disease. Cancers (Basel) 13(21). https://doi.org/10.3390/cancers13215572
Hill JA, Zamora D, Xie H, Thur LA, Delaney C, Dahlberg A, Pergam SA, Leisenring WM, Boeckh M, Milano F (2021) Delayed-onset cytomegalovirus infection is frequent after discontinuing letermovir in cord blood transplant recipients. Blood Adv 5(16):3113–3119. https://doi.org/10.1182/bloodadvances.2021004362
Derigs P, Radujkovic A, Schubert ML, Schnitzler P, Schöning T, Müller-Tidow C, Hegenbart U, Schönland SO, Luft T, Dreger P, Schmitt M (2021) Letermovir prophylaxis is effective in preventing cytomegalovirus reactivation after allogeneic hematopoietic cell transplantation: single-center real-world data. Ann Hematol 100(8):2087–2093. https://doi.org/10.1007/s00277-020-04362-2
Serio B, Giudice V, Guariglia R, Fontana R, Pezzullo L, Martorelli MC, Ferrara I, Mettivier L, D’Addona M, Vaccaro E, Langella M, Selleri C (2021) Prophylactic letermovir decreases cytomegalovirus reactivation after stem cell transplantation: a single-center real-world evidence study. Infez Med 29(1):102–113
Lin A, Flynn J, DeRespiris L, Figgins B, Griffin M, Lau C, Proli A, Devlin SM, Cho C, Tamari R, Jakubowski AA, Papadopoulos EB, Giralt SA, Perales MA, Seo SK, Shaffer B (2021) Letermovir for prevention of cytomegalovirus reactivation in haploidentical and mismatched adult donor allogeneic hematopoietic cell transplantation with post-transplantation cyclophosphamide for graft-versus-host disease prophylaxis. Transplant Cell Ther 27(1):85.e81–85.e86. https://doi.org/10.1016/j.bbmt.2020.10.009
Johnsrud JJ, Nguyen IT, Domingo W, Narasimhan B, Efron B, Brown JW (2020) Letermovir prophylaxis decreases burden of cytomegalovirus (CMV) in patients at high risk for CMV disease following hematopoietic cell transplant. Biol Blood Marrow Transplant 26(10):1963–1970. https://doi.org/10.1016/j.bbmt.2020.07.002
Anderson A, Raja M, Vazquez N, Morris M, Komanduri K, Camargo J (2020) Clinical “real-world” experience with letermovir for prevention of cytomegalovirus infection in allogeneic hematopoietic cell transplant recipients. Clin Transpl 34(7):e13866. https://doi.org/10.1111/ctr.13866
Sharma P, Gakhar N, MacDonald J, Abidi MZ, Benamu E, Bajrovic V, Purev E, Haverkos BM, Tobin J, Kaiser J, Chase S, Miller M, Weinberg A, Gutman JA (2020) Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation. Bone Marrow Transplant 55(4):780–786. https://doi.org/10.1038/s41409-019-0730-y
Vyas A, Raval AD, Kamat S, LaPlante K, Tang Y, Chemaly RF (2023) Real-world outcomes associated with letermovir use for cytomegalovirus primary prophylaxis in allogeneic hematopoietic cell transplant recipients: a systematic review and meta-analysis of observational studies. Open Forum Infect Dis 10(1):ofac687. https://doi.org/10.1093/ofid/ofac687
Mori Y, Jinnouchi F, Takenaka K, Aoki T, Kuriyama T, Kadowaki M, Odawara J, Ueno T, Kohno K, Harada T, Yoshimoto G, Takase K, Henzan H, Kato K, Ito Y, Kamimura T, Ohno Y, Ogawa R, Eto T et al (2021) Efficacy of prophylactic letermovir for cytomegalovirus reactivation in hematopoietic cell transplantation: a multicenter real-world data. Bone Marrow Transplant 56(4):853–862. https://doi.org/10.1038/s41409-020-01082-z
Atsuta Y (2016) Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol 103(1):3–10. https://doi.org/10.1007/s12185-015-1894-x
Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow. Transplantation 11(12):945–956. https://doi.org/10.1016/j.bbmt.2005.09.004
Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, Storer B (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106(8):2912–2919. https://doi.org/10.1182/blood-2005-05-2004
Ljungman P, Schmitt M, Marty FM, Maertens J, Chemaly RF, Kartsonis NA, Butterton JR, Wan H, Teal VL, Sarratt K, Murata Y, Leavitt RY, Badshah C (2020) A mortality analysis of letermovir prophylaxis for cytomegalovirus (CMV) in CMV-seropositive recipients of allogeneic hematopoietic cell transplantation. Clin Infect Dis 70(8):1525–1533. https://doi.org/10.1093/cid/ciz490
Ljungman P, Griffiths P, Paya C (2002) Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis 34(8):1094–1097
Gray RJ (1988) A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 16:1141–1154
Fine JP, Gray RJ (1999) A proportional hazards model for subdistribution of a competing risk. J Am Stat Assoc 94:456–509
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Malagola M, Pollara C, Polverelli N, Zollner T, Bettoni D, Gandolfi L, Gramegna D, Morello E, Turra A, Corbellini S, Signorini L, Moioli G, Bernardi S, Zanaglio C, Farina M, Testa TE, Caruso A, Russo D (2020) Advances in CMV management: a single center real-life experience. Front Cell Dev Biol 8:534268. https://doi.org/10.3389/fcell.2020.534268
Royston L, Royston E, Masouridi-Levrat S, Vernaz N, Chalandon Y, Van Delden C, Neofytos D (2021) Letermovir primary prophylaxis in high-risk hematopoietic cell transplant recipients: a matched cohort study. Vaccines (Basel) 9(4). https://doi.org/10.3390/vaccines9040372
Royston L, Royston E, Masouridi-Levrat S, Chalandon Y, Van Delden C, Neofytos D (2021) Predictors of breakthrough clinically significant cytomegalovirus infection during letermovir prophylaxis in high-risk hematopoietic cell transplant recipients. Immun Inflamm Dis 9(3):771–776. https://doi.org/10.1002/iid3.431
Sassine J, Khawaja F, Shigle TL, Handy V, Foolad F, Aitken SL, Jiang Y, Champlin R, Shpall E, Rezvani K, Ariza-Heredia EJ, Chemaly RF (2021) Refractory and resistant cytomegalovirus after hematopoietic cell transplant in the letermovir primary prophylaxis era. Clin Infect Dis 73(8):1346–1354. https://doi.org/10.1093/cid/ciab298
Gabanti E, Borsani O, Colombo AA, Zavaglio F, Binaschi L, Caldera D, Sciarra R, Cassinelli G, Alessandrino EP, Bernasconi P, Ferretti VV, Lilleri D, Baldanti F (2022) Human cytomegalovirus-specific T-cell reconstitution and late-onset cytomegalovirus infection in hematopoietic stem cell transplantation recipients following letermovir prophylaxis. Transplant Cell Ther 28(4):211.e211–211.e219. https://doi.org/10.1016/j.jtct.2022.01.008
Dadwal S, Russo D, Stelljes M, Schmitt M, Pilorge S, Teal V, Haber B, Bopp C, Badshah C (2023) A phase 3 randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of letermovir (LET) prophylaxis when extended from 100 to 200 days post-transplant in cytomegalovirus (CMV)-seropositive recipients (R+) of an allogeneic hematopoietic stem cell transplant (HSCT). Transplant Cell Ther 29(2, supplement):S64 (meeting abstract). https://doi.org/10.1016/S2666-6367(23)00145-8
de la Camara R (2016) CMV in hematopoietic stem cell transplantation. Mediterr J Hematol Infect Dis 8(1):e2016031. https://doi.org/10.4084/MJHID.2016.031
Acknowledgements
The authors are grateful for the work of all the physicians and data managers at the centers that contributed valuable data on transplantation to the Japanese Society for Transplantation and Cellular Therapy (JSTCT). They also thank all the members of the Transplant Registry Unified Management committees at JSTCT for their dedicated data management.
Funding
This work was partially supported by JSPS KAKENHI Grant Number 18H02840.
Author information
Authors and Affiliations
Contributions
KT, SF, TM, MO, SS, KM, and HN designed the study. KT performed the statistical analyses and wrote the manuscript. NO, TK, MT, TA, KI, YO, YK, MS, YM, TF, and HN provided patient data. TK and YA collected patient data. All authors interpreted the data and reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the data management committee of the JSTCT and by the ethics committee of the Ehime University School of Medicine. All procedures performed in studies were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
K.T. has received a speaker honoraria from MSD, outside the submitted work. Other authors declare no competing financial interests regarding this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Takenaka, K., Fuji, S., Matsukawa, T. et al. Outcomes of allogeneic hematopoietic cell transplantation under letermovir prophylaxis for cytomegalovirus infection. Ann Hematol 103, 285–296 (2024). https://doi.org/10.1007/s00277-023-05474-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05474-1