Abstract
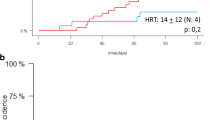
Graft failure and delayed hematopoietic recovery are the major limitations of cord-blood transplantation (CBT). Romiplostim, a thrombopoietin-receptor agonist, promotes megakaryopoiesis and multilineage hematopoiesis in aplastic anemia. The decreased number of hematopoietic stem cells in the early phase after CBT and aplastic anemia share certain characteristics. Therefore, we hypothesized that romiplostim administration immediately after CBT may promote multilineage hematopoietic recovery. We investigated the safety and preliminary efficacy of administering romiplostim a day after CBT. This phase 1 dose-escalation study included six adults with hematologic malignancies in remission. Romiplostim was administered subcutaneously within 7 days after single-unit CBT, initially at doses of 5 µg/kg or 10 µg/kg in three patients, then once a week for 14 weeks or until platelet recovery. The maximum dose was 20 µg/kg. The median number of romiplostim administrations was 6 (range, 3–15). Romiplostim-related adverse events included bone pain (3/6) and injection site reaction (1/6). Non-hematological grade ≥ 3 toxicities were observed in four patients; febrile neutropenia was the most common (4/6). All patients achieved neutrophil engraftment and the median time was 14 days (range, 12–32). Platelet counts ≥ 50 × 109 /L were recorded in all patients except for one who died on day 48; the median time was 34 days (range, 29–98). No relapse, thrombosis, or bone marrow fibrosis was observed during a median follow-up of 34 months. Romiplostim may be safely administered in the early phase of CBT. Further phase 2 trial is warranted for its efficacy evaluation. Trial registration number: UMIN000033799, August 18, 2018.


Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author upon reasonable request.
References
Gluckman E, Rocha V (2009) Cord blood transplantation: state of the art. Haematologica 94:451–454. https://doi.org/10.3324/haematol.2009.005694
Eapen M, Rocha V, Sanz G et al (2010) Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 11:653–660. https://doi.org/10.1016/s1470-2045(10)70127-3
Kurita N, Gosho M, Yokoyama Y et al (2017) A phase I/II trial of intrabone marrow cord blood transplantation and comparison of the hematological recovery with the Japanese nationwide database. Bone Marrow Transplant 52:574–579. https://doi.org/10.1038/bmt.2016.319
Labrador J, Lopez-Anglada L, Perez-Lopez E et al (2013) Analysis of incidence, risk factors and clinical outcome of thromboembolic and bleeding events in 431 allogeneic hematopoietic stem cell transplantation recipients. Haematologica 98:437–443. https://doi.org/10.3324/haematol.2012.069559
Solh M, Brunstein C, Morgan S, Weisdorf D (2011) Platelet and red blood cell utilization and transfusion independence in umbilical cord blood and allogeneic peripheral blood hematopoietic cell transplants. Biol Blood Marrow Transplant 17:710–716. https://doi.org/10.1016/j.bbmt.2010.08.017
Ramírez P, Brunstein CG, Miller B, Defor T, Weisdorf D (2011) Delayed platelet recovery after allogeneic transplantation: a predictor of increased treatment-related mortality and poorer survival. Bone Marrow Transplant 46:981–986. https://doi.org/10.1038/bmt.2010.218
Akahoshi Y, Kimura SI, Gomyo A et al (2018) Delayed platelet recovery after allogeneic hematopoietic stem cell transplantation: association with chronic graft-versus-host disease and survival outcome. Hematol Oncol 36:276–284. https://doi.org/10.1002/hon.2427
Kaushansky K, Lok S, Holly RD et al (1994) Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature 369:568–571. https://doi.org/10.1038/369568a0
Nishikii H, Kanazawa Y, Umemoto T et al (2015) Unipotent Megakaryopoietic pathway bridging hematopoietic stem cells and mature megakaryocytes. Stem Cells 33:2196–2207. https://doi.org/10.1002/stem.1985
Peffault de Latour R, Chevret S, Ruggeri AL et al (2020) Romiplostim in patients undergoing hematopoietic stem cell transplantation: results of a phase 1/2 multicenter trial. Blood 135:227–229. https://doi.org/10.1182/blood.2019000358
Ahmed S, Bashir Q, Bassett R et al (2021) Eltrombopag for post-transplantation thrombocytopenia: results of phase II randomized, double-blind, placebo-controlled trial. Transplant Cell Ther 27:430.e1-430.e7. https://doi.org/10.1016/j.jtct.2021.02.004
Christakopoulos GE, DeFor TE, Hage S, Wagner JE, Linden MA, Brunstein C, Bejanyan N, Verneris MR, Smith AR (2021) Phase I dose-finding, safety, and tolerability trial of romiplostim to improve platelet recovery after UCB transplantation. Transplant Cell Ther 27:497.e1-497.e6. https://doi.org/10.1016/j.jtct.2021.02.033
Mahat U, Rotz SJ, Hanna R (2020) Use of thrombopoietin receptor agonists in prolonged thrombocytopenia after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 26:e65–e73. https://doi.org/10.1016/j.bbmt.2019.12.003
Tang B, Huang L, Liu H et al (2020) Recombinant human thrombopoietin promotes platelet engraftment after umbilical cord blood transplantation. Blood Adv 4:3829–3839. https://doi.org/10.1182/bloodadvances.2020002257
Kimura S, Roberts AW, Metcalf D, Alexander WS (1998) Hematopoietic stem cell deficiencies in mice lacking c-Mpl, the receptor for thrombopoietin. Proc Natl Acad Sci U S A 95:1195–1200. https://doi.org/10.1073/pnas.95.3.1195
Jang JH, Tomiyama Y, Miyazaki K et al (2021) Efficacy and safety of romiplostim in refractory aplastic anaemia: a phase II/III, multicentre, open-label study. Br J Haematol 192:190–199. https://doi.org/10.1111/bjh.17190
Olnes MJ, Scheinberg P, Calvo KR et al (2012) Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med 367:11–19. https://doi.org/10.1056/nejmoa1200931
Huo Y, Wu L, Pang A et al (2023) Single-cell dissection of human hematopoietic reconstitution after allogeneic hematopoietic stem cell transplantation. Sci Immunol 8:eabn6429. https://doi.org/10.1126/sciimmunol.abn6429
Miyawaki K, Iwasaki H, Jiromaru T et al (2017) Identification of unipotent megakaryocyte progenitors in human hematopoiesis. Blood 129:3332–3343. https://doi.org/10.1182/blood-2016-09-741611
Pasvolsky O, Shargian L, Rozovski U et al (2021) Eltrombopag for enhancement of platelet engraftment in patients undergoing allogeneic cord blood transplantation. Leuk Lymphoma 62:2747–2754. https://doi.org/10.1080/10428194.2021.1929957
Chan AW, Tetzlaff JM, Altman DG et al (2015) SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Ann Intern Med 158:200–207. https://doi.org/10.7326/0003-4819-158-3-201302050-00583
Bacigalupo A, Ballen K, Rizzo D et al (2009) Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant 15:1628–1633. https://doi.org/10.1016/j.bbmt.2009.07.004
Carson JL, Guyatt G, Heddle NM et al (2016) Clinical practice guidelines from the AABB: red blood cell transfusion thresholds and storage. JAMA 316:2025–2035. https://doi.org/10.1001/jama.2016.9185
Kaufman RM, Djulbegovic B, Gernsheimer T et al (2015) Platelet transfusion: a clinical practice guideline from the AABB. Ann Intern Med 162:205–213. https://doi.org/10.7326/m14-1589
Bearman SI, Appelbaum FR, Buckner CD, Petersen FB, Fisher LD, Clift RA, Thomas ED (1988) Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol 6:1562–1568. https://doi.org/10.1200/jco.1988.6.10.1562
Spitzer TR (2001) Engraftment syndrome following hematopoietic stem cell transplantation. Bone Marrow Transplant 27:893–898. https://doi.org/10.1038/sj.bmt.1703015
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus conference on acute GVHD grading. Bone Marrow Transplant 15:825–828
Filipovich AH, Weisdorf D, Pavletic S et al (2005) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 11:945–956. https://doi.org/10.1016/j.bbmt.2005.09.004
Rebulla P, Finazzi G, Marangoni F, Avvisati G, Gugliotta L, Tognoni G, Barbui T, Mandelli F, Sirchia G (1997) The threshold for prophylactic platelet transfusions in adults with acute myeloid leukemia. Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto. N Engl J Med 337:1870–1875. https://doi.org/10.1056/nejm199712253372602
Liesveld JL, Phillips GL, Becker M et al (2013) A phase 1 trial of eltrombopag in patients undergoing stem cell transplantation after total body irradiation. Biol Blood Marrow Transplant 19:1745–1752. https://doi.org/10.1016/j.bbmt.2013.10.002
Han TT, Xu LP, Liu DH et al (2015) Recombinant human thrombopoietin promotes platelet engraftment after haploidentical hematopoietic stem cell transplantation: a prospective randomized controlled trial. Ann Hematol 94:117–128. https://doi.org/10.1007/s00277-014-2158-1
Wen B, Zhang X, Chen S, Fan J, Yang S, Cai Y, Wang P, Zhang Q, Gu Q, Du X (2022) Oral eltrombopag versus subcutaneous recombinant human thrombopoietin for promoting platelet engraftment after allogeneic stem cell transplantation: a prospective, non-inferiority, randomized controlled trial. Hematol Oncol 40:777–786. https://doi.org/10.1002/hon.3017
Li J, Yang C, Xia Y, Bertino A, Glaspy J, Roberts M, Kuter DJ (2001) Thrombocytopenia caused by the development of antibodies to thrombopoietin. Blood 98:3241–3248. https://doi.org/10.1182/blood.v98.12.3241
Konuma T, Kohara C, Watanabe E, Mizukami M, Nagai E, Oiwa-Monna M, Tanoue S, Isobe M, Kato S, Tojo A, Takahashi S (2017) Cytokine profiles of pre-engraftment syndrome after single-unit cord blood transplantation for adult patients. Biol Blood Marrow Transplant 23:1932–1938. https://doi.org/10.1016/j.bbmt.2017.07.020
Terakura S, Wake A, Inamoto Y et al (2017) Exploratory research for optimal GvHD prophylaxis after single unit CBT in adults: short-term methotrexate reduced the incidence of severe GvHD more than mycophenolate mofetil. Bone Marrow Transplant 52:423–430. https://doi.org/10.1038/bmt.2016.255
Terakura S, Kuwatsuka Y, Yamasaki S et al (2017) GvHD prophylaxis after single-unit reduced intensity conditioning cord blood transplantation in adults with acute leukemia. Bone Marrow Transplant 52:1261–1267. https://doi.org/10.1038/bmt.2017.116
Lambertini M, Del Mastro L, Bellodi A, Pronzato P (2014) The five “Ws” for bone pain due to the administration of granulocyte-colony stimulating factors (G-CSFs). Crit Rev Oncol Hematol 89:112–128. https://doi.org/10.1016/j.critrevonc.2013.08.006
Yamaguchi M, Hirouchi T, Yokoyama K, Nishiyama A, Murakami S, Kashiwakura I (2018) The thrombopoietin mimetic romiplostim leads to the complete rescue of mice exposed to lethal ionizing radiation. Sci Rep 8:10659. https://doi.org/10.1038/s41598-018-29013-5
Konuma T, Kanda J, Yamasaki S et al (2021) Single cord blood transplantation versus unmanipulated haploidentical transplantation for adults with acute myeloid leukemia in complete remission. Transplant Cell Ther 27:334.e1-334.e11. https://doi.org/10.1016/j.jtct.2021.01.023
Nakasone H, Fuji S, Yakushijin K et al (2017) Impact of total body irradiation on successful neutrophil engraftment in unrelated bone marrow or cord blood transplantation. Am J Hematol 92:171–178. https://doi.org/10.1002/ajh.24613
Acknowledgements
This study was supported by the Grant for Implementation of Advanced Medicine at the University of Tsukuba Hospital. The authors would like to thank the study participants. This study received generous support from Toshihiro Kikuchi in designing the trial. We thank all staff of the Tsukuba Clinical Research and Development Organization (T-CReDO) for their invaluable assistance with data collection and quality management. We thank Editage (www.editage.jp) for the grammatical review and advice.
Funding
This study was supported by the Grant for Implementation of Advanced Medicine at the University of Tsukuba Hospital.
Author information
Authors and Affiliations
Contributions
NK designed and conducted the study, interpreted the data, and wrote the manuscript. HN, YM, YS, KH, TS, TK, YY, NO, and MS-Y conducted the study and reviewed the manuscript. KM, TO, HY, TI, HM, and KH performed data collection and quality management. RM performed the pathological examination. The first draft of the manuscript was written by NK and all authors commented on previous versions of the manuscript. SC designed and supervised the project and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the institutional review board of University of Tsukuba Hospital (approval #I-27). UMIN Clinical Trials Registry identifier: UMIN000033799.
Consent
Written informed consent was obtained from all patients in accordance with the Declaration of Helsinki.
Competing interests
The study was sponsored by Kyowa Kirin Co., Ltd., which provided the study drug to the participants free of charge. The authors declare that no funding or sponsoring agency played any role in the study design, patient enrollment, data acquisition/analysis, manuscript drafting, or the decision to publish this study. MS-Y received research funding from Eisai, Bristol Myers Squibb, and Otsuka. SC received research funding from Kyowa Kirin, Chugai, Ono, Astellas, Beyer, Eisai, and Thyas. The other authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kurita, N., Nishikii, H., Maruyama, Y. et al. Safety of romiplostim administered immediately after cord-blood transplantation: a phase 1 trial. Ann Hematol 102, 2895–2902 (2023). https://doi.org/10.1007/s00277-023-05410-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05410-3