Abstract
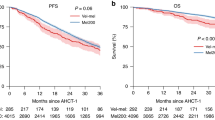
Despite the development of effective agents for multiple myeloma (MM), the management of patients with high-risk MM (HRMM) is challenging. High-dose treatment followed by autologous stem cell transplantation (ASCT) is regarded as upfront treatment for transplant-eligible patients with HRMM. In the present study, we retrospectively investigated the efficacies of two conditioning regimens for upfront ASCT in newly diagnosed patients with MM and high-risk features: high-dose melphalan (HDMEL; 200 mg/m2) and busulfan plus melphalan (BUMEL). In total, 221 patients underwent ASCT between May 2005 and June 2021; among these 221 patients, 79 had high-risk cytogenetic abnormalities. In patients with high-risk cytogenetics, BUMEL showed a tendency toward longer overall survival (OS) and progression-free survival (PFS) compared to HDMEL (median OS; not reached vs. 53.2 months; P = 0.091, median PFS; not reached vs. 31.7 months; P = 0.062). Additionally, multivariate analysis revealed that BUMEL was significantly associated with PFS (hazard ratio = 0.37, 95% confidence interval = 0.15–0.89, P = 0.026). We compared BUMEL with HDMEL in patients with other high-risk features, such as high lactate dehydrogenase level, extramedullary disease, and poor response to frontline therapy. Notably, among patients with less than very good partial response (VGPR) to frontline therapy, median PFS was significantly longer in the BUMEL group than in the HDMEL group (55.1 vs. 17.3 months, respectively; P = 0.011). These findings indicate that BUMEL may be an effective conditioning regimen for upfront ASCT in MM patients with high-risk cytogenetics; BUMEL may be more appropriate than HDMEL for patients with less than VGPR to frontline therapy.


Similar content being viewed by others
Availability of data and materials
Data from this study can be obtained from the corresponding author on reasonable request.
References
Raza S, Safyan RA, Rosenbaum E, Bowman AS, Lentzsch S (2017) Optimizing current and emerging therapies in multiple myeloma: a guide for the hematologist. Ther Adv Hematol 8(2):55–70
Brenner H, Gondos A, Pulte D (2008) Recent major improvement in long-term survival of younger patients with multiple myeloma. Blood 111(5):2521–2526
Lahuerta JJ, Grande C, Blade J, Martínez-López J, de la Serna J, Alegre A, Garcia LJ, Caballero D, de la Rubia J, Marín J et al (2002) Myeloablative treatments for multiple myeloma: update of a comparative study of different regimens used in patients from the Spanish registry for transplantation in multiple myeloma. Leuk Lymphoma 43(1):67–74
Ria R, Falzetti F, Ballanti S, Minelli O, Di Ianni M, Cimminiello M, Vacca A, Dammacco F, Martelli MF, Tabilio A (2004) Melphalan versus melphalan plus busulphan in conditioning to autologous stem cell transplantation for low-risk multiple myeloma. Hematol J 5(2):118–122
Chanan-Khan AA, Giralt S (2010) Importance of achieving a complete response in multiple myeloma, and the impact of novel agents. J Clin Oncol 28(15):2612–2624
Nooka AK, Kaufman JL, Muppidi S, Langston A, Heffner LT, Gleason C, Casbourne D, Saxe D, Boise LH, Lonial S (2014) Consolidation and maintenance therapy with lenalidomide, bortezomib and dexamethasone (RVD) in high-risk myeloma patients. Leukemia 28(3):690–693
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, Arnulf B, Macro M, Belhadj K, Garderet L et al (2017) Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med 376(14):1311–1320
Durie BGM, Hoering A, Abidi MH, Rajkumar SV, Epstein J, Kahanic SP, Thakuri M, Reu F, Reynolds CM, Sexton R et al (2017) Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): a randomised, open-label, phase 3 trial. Lancet 389(10068):519–527
Rajkumar SV (2022) Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. American Journal of Hematology 97(8):1086–1107
Roussel M, Moreau P, Huynh A, Mary JY, Danho C, Caillot D, Hulin C, Fruchart C, Marit G, Pégourié B et al (2010) Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM). Blood 115(1):32–37
Bashir Q, Thall PF, Milton DR, Fox PS, Kawedia JD, Kebriaei P, Shah N, Patel K, Andersson BS, Nieto YL et al (2019) Conditioning with busulfan plus melphalan versus melphalan alone before autologous haemopoietic cell transplantation for multiple myeloma: an open-label, randomised, phase 3 trial. Lancet Haematol 6(5):e266–e275
Roussel M, Lauwers-Cances V, Macro M, Leleu X, Royer B, Hulin C, Karlin L, Perrot A, Touzeau C, Chrétien ML et al (2022) Bortezomib and high-dose melphalan conditioning regimen in frontline multiple myeloma: an IFM randomized phase 3 study. Blood 139(18):2747–2757
Saini N, Bashir Q, Milton DR, Tang G, Delgado R, Rondon G, Popat UR, Hosing CM, Nieto Y, Kebriaei P et al (2020) Busulfan and melphalan conditioning is superior to melphalan alone in autologous stem cell transplantation for high-risk MM. Blood Adv 4(19):4834–4837
Haas PS, Bauchmüller K, Kühnemund A, Finke J, Ihorst G, Engelhardt M (2006) Efficacy of diverse high-dose regimens followed by autologous peripheral blood stem cell transplantation in consecutive multiple myeloma patients: a single-centre analysis over a 12-year period. Ann Hematol 85(3):191–193
Song GY, Jung SH, Kim JS, Eom HS, Moon JH, Yhim HY, Kim K, Min CK, Lee JJ (2022) Busulfan and thiotepa as a conditioning regimen for autologous stem cell transplantation in patients with multiple myeloma: A study of the Korean Multiple Myeloma Working Party (KMMWP-1801 study). Front Oncol 12:959949
Park SS, Kim K, Kim SJ, Lee JH, Yoon SS, Mun YC, Lee JJ, Eom HS, Kim JS, Min CK (2019) A Phase I/II, Open-Label, Prospective, Multicenter Study to Evaluate the Efficacy and Safety of Lower Doses of Bortezomib Plus Busulfan and Melphalan as a Conditioning Regimen in Patients with Multiple Myeloma Undergoing Autologous Peripheral Blood Stem Cell Transplantation: The KMM103 Study. Biol Blood Marrow Transplant 25(7):1312–1319
Nieto Y, Valdez BC, Pingali SR, Bassett R, Delgado R, Nguyen J, Shah N, Popat U, Jones RB, Andersson BS et al (2017) High-dose gemcitabine, busulfan, and melphalan for autologous stem-cell transplant in patients with relapsed or refractory myeloma: a phase 2 trial and matched-pair comparison with melphalan. Lancet Haematol 4(6):e283–e292
Talamo G, Claxton DF, Dougherty DW, Ehmann CW, Sivik J, Drabick JJ, Rybka W (2009) BU and CY as conditioning regimen for autologous transplant in patients with multiple myeloma. Bone Marrow Transplant 44(3):157–161
Abidi MH, Agarwal R, Ayash L, Deol A, Al-Kadhimi Z, Abrams J, Cronin S, Ventimiglia M, Lum L, Zonder J et al (2012) Melphalan 180 mg/m2 can be safely administered as conditioning regimen before an autologous stem cell transplantation (ASCT) in multiple myeloma patients with creatinine clearance 60 mL/min/1.73 m2 or lower with use of palifermin for cytoprotection: results of a phase I trial. Biol Blood Marrow Transplant 18(9):1455–1461
Blanes M, Lahuerta JJ, González JD, Ribas P, Solano C, Alegre A, Bladé J, San Miguel JF, Sanz MA, de la Rubia J (2013) Intravenous busulfan and melphalan as a conditioning regimen for autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: a matched comparison to a melphalan-only approach. Biol Blood Marrow Transplant 19(1):69–74
Moreau P, Facon T, Attal M, Hulin C, Michallet M, Maloisel F, Sotto JJ, Guilhot F, Marit G, Doyen C et al (2002) Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood 99(3):731–735
Rajkumar SV, Harousseau JL, Durie B, Anderson KC, Dimopoulos M, Kyle R, Blade J, Richardson P, Orlowski R, Siegel D et al (2011) Consensus recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. Blood 117(18):4691–4695
Sevcikova S, Minarik J, Stork M, Jelinek T, Pour L, Hajek R (2019) Extramedullary disease in multiple myeloma - controversies and future directions. Blood Rev 36:32–39
Kanda Y (2013) Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 48(3):452–458
Mateos MV, Martínez BP, González-Calle V (2021) High-risk multiple myeloma: how to treat at diagnosis and relapse? Hematology Am Soc Hematol Educ Program 2021(1):30–36
Sonneveld P, Avet-Loiseau H, Lonial S, Usmani S, Siegel D, Anderson KC, Chng WJ, Moreau P, Attal M, Kyle RA et al (2016) Treatment of multiple myeloma with high-risk cytogenetics: a consensus of the International Myeloma Working Group. Blood 127(24):2955–2962
D'Agostino M, Cairns DA, Lahuerta JJ, Wester R, Bertsch U, Waage A, Zamagni E, Mateos M-V, Dall'Olio D, Van De Donk NW et al (2022) Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J Clin Oncol 40(29):3406–3418
Mangiacavalli S, Pompa A, Ferretti V, Klersy C, Cocito F, Varettoni M, Cartia CS, Cazzola M, Corso A (2017) The possible role of burden of therapy on the risk of myeloma extramedullary spread. Ann Hematol 96(1):73–80
Vij R, Kumar S, Zhang MJ, Zhong X, Huang J, Dispenzieri A, Abidi MH, Bird JM, Freytes CO, Gale RP et al (2015) Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant 21(2):335–341
Lahuerta JJ, Martinez-Lopez J, Grande C, Bladé J, Serna JDL, Alegre A, García-Laraña J, Caballero D, Sureda A, Rubia JDL et al (2000) Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol 109(1):138–147
Park S, Shin DY, Hong J, Kim I, Koh Y, Byun JM, Yoon SS (2021) Busulfan plus melphalan versus melphalan alone conditioning regimen after bortezomib based triplet induction chemotherapy for patients with newly diagnosed multiple myeloma. Ther Adv Hematol 12:20406207211012985
Acknowledgments
None
Author information
Authors and Affiliations
Contributions
JSH and JJL designed the study; MK and SHJ prepared the manuscript; CKM, JYL, JCJ, SSY, SNL, YRD, KK, JHL, KHY, SHB, JHY, JJ, and HSE critically reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
This study was approved by the institutional ethics committees of participating institutions and was conducted in accordance with the Declaration of Helsinki. The institutional ethics committees waived the requirement for informed patient consent because of the retrospective study design.
Consent for publication
Not applicable.
Conflicts of interests
The authors have no conflicts of interest to declare.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 16 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Lee, JJ., Min, CK. et al. Busulfan plus melphalan versus high-dose melphalan as a conditioning regimen for autologous stem cell transplantation in multiple myeloma with high-risk features (KMM 2015). Ann Hematol 102, 2233–2240 (2023). https://doi.org/10.1007/s00277-023-05308-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05308-0