Abstract
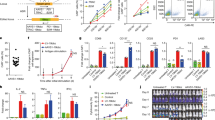
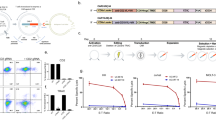
Chimeric antigen receptor T cell therapy (CAR-T) is a novel treatment that has produced unprecedented clinical effects in patients with hematological malignancies. Acute adverse events often occur following adoptive immunotherapy. Therefore, a suicide gene is helpful, which is a genetically encoded mechanism that allows selective destruction of adoptively transferred T cells in the face of unacceptable toxicity. RQR8 is a gene that integrates CD34 and CD20 epitopes. In our study, we incorporated the suicide gene RQR8 into CAR-T cells, so it enabled rituximab to eliminate vector/transgene-expressing T cells via antibody-dependent cell-mediated cytotoxicity and complement dependent cytotoxicity. In this work, we explored the functionality of RQR8 CAR-T cells in vitro and in vivo. We believe that RQR8 as a safety switch will make CAR-T cell therapy safer and less costly.






Similar content being viewed by others
Abbreviations
- CAR-T cell:
-
Chimeric antigen receptor T cell
- CLL1:
-
C type lectin domain family 12 member A
- NK cell:
-
Natural killer cell
- MOI:
-
Multiplicity of infection
- NSG:
-
NOD-Prkdcscid Il2rgtm1
- CRS:
-
Cytokine release syndrome
- ICANS:
-
Immune effector cell associated neurotoxic syndrome
- HSV-TK:
-
Herpes simplex virus thymidine kinase
- iCasp9:
-
Inducible caspase 9
- ADCC:
-
Antibody-dependent cell-mediated cytotoxicity
- CDC:
-
Complement dependent toxicity
- MND:
-
A myeloproliferative sarcoma virus enhancer, a dl587Rev primer binding site substituted promoter
- EF-1α:
-
Extension factor 1α
References
June CH, Sadelain M (2018) Chimeric antigen receptor therapy. N Engl J Med 379(1):64–73. https://doi.org/10.1056/NEJMra1706169
Lee DW, Kochenderfer JN, Stetler-Stevenson M et al (2015) T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet (London, England) 385(9967):517–528. https://doi.org/10.1016/s0140-6736(14)61403-3
Fitzgerald JC, Weiss SL, Maude SL et al (2017) Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit Care Med 45(2):e124-ee31. https://doi.org/10.1097/ccm.0000000000002053
Schubert ML, Schmitt M, Wang L et al (2021) Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann Oncol 32(1):34–48. https://doi.org/10.1016/j.annonc.2020.10.478
Yu S, Yi M, Qin S et al (2019) Next generation chimeric antigen receptor T cells: safety strategies to overcome toxicity. Mol Cancer 18(1):125. https://doi.org/10.1186/s12943-019-1057-4
Maude SL, Frey N, Shaw PA et al (2014) Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 371(16):1507–1517. https://doi.org/10.1056/NEJMoa1407222
Porter DL, Hwang WT, Frey NV et al (2015) Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 7(303):303ra139. https://doi.org/10.1126/scitranslmed.aac5415
Ali SA, Shi V, Maric I et al (2016) T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood 128(13):1688–1700. https://doi.org/10.1182/blood-2016-04-711903
Davila ML, Riviere I, Wang X et al (2014) Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 6(224):224ra25. https://doi.org/10.1126/scitranslmed.3008226
Neelapu SS, Tummala S, Kebriaei P et al (2018) Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat Rev Clin Oncol 15(1):47–62. https://doi.org/10.1038/nrclinonc.2017.148
Zhang H, Hu Y, Shao M et al (2021) Dasatinib enhances anti-leukemia efficacy of chimeric antigen receptor T cells by inhibiting cell differentiation and exhaustion. J Hematol 14(1):113. https://doi.org/10.1186/s13045-021-01117-y
Zheng Y, Nandakumar KS, Cheng K (2021) Optimization of CAR-T cell-based therapies using small-molecule-based safety switches. J Med Chem 64(14):9577–9591. https://doi.org/10.1021/acs.jmedchem.0c02054
Hotblack A, Kokalaki EK, Palton MJ et al (2021) Tunable control of CAR T cell activity through tetracycline mediated disruption of protein-protein interaction. Sci Rep 11(1):21902. https://doi.org/10.1038/s41598-021-01418-9
Marin V, Cribioli E, Philip B et al (2012) Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods 23(6):376–386. https://doi.org/10.1089/hgtb.2012.050
Yi QY, Bai ZS, Cai B et al (2018) HSV-TK/GCV can induce cytotoxicity of retinoblastoma cells through autophagy inhibition by activating MAPK/ERK. Onco Rep 40(2):682–692. https://doi.org/10.3892/or.2018.6454
Straathof KC, Pulè MA, Yotnda P et al (2005) An inducible caspase 9 safety switch for T-cell therapy. Blood 105(11):4247–4254. https://doi.org/10.1182/blood-2004-11-4564
Gargett T, Brown MP (2014) The inducible caspase-9 suicide gene system as a "safety switch" to limit on-target, off-tumor toxicities of chimeric antigen receptor T cells. Front Pharmacol 5:235. https://doi.org/10.3389/fphar.2014.00235
Di Stasi A, Tey SK, Dotti G et al (2011) Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med 365(18):1673–1683. https://doi.org/10.1056/NEJMoa1106152
Philip B, Kokalaki E, Mekkaoui L et al (2014) A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124(8):1277–1287. https://doi.org/10.1182/blood-2014-01-545020
Mosti L, Langner LM, Chmielewski KO et al (2021) Targeted multi-epitope switching enables straightforward positive/negative selection of CAR T cells. Gene Ther 28(9):602–612. https://doi.org/10.1038/s41434-021-00220-6
Binder M, Otto F, Mertelsmann R et al (2006) The epitope recognized by rituximab. Blood 108(6):1975–1978. https://doi.org/10.1182/blood-2006-04-014639
Gargett T, Truong N, Ebert LM et al (2019) Optimization of manufacturing conditions for chimeric antigen receptor T cells to favor cells with a central memory phenotype. Cytotherapy 21(6):593–602. https://doi.org/10.1016/j.jcyt.2019.03.003
Hinrichs CS, Borman ZA, Gattinoni L et al (2011) Human effector CD8+ T cells derived from naive rather than memory subsets possess superior traits for adoptive immunotherapy. Blood 117(3):808–814. https://doi.org/10.1182/blood-2010-05-286286
Jin X, Lu W, Zhang M et al (2021) Infection temperature affects the phenotype and function of chimeric antigen receptor T cells produced via lentiviral technology. Front Immunol 12:638907. https://doi.org/10.3389/fimmu.2021.638907
Neelapu SS, Locke FL, Bartlett NL et al (2017) Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 377(26):2531–2544. https://doi.org/10.1056/NEJMoa1707447
Wang Z, Wu Z, Liu Y et al (2017) New development in CAR-T cell therapy. J Hematol 10(1):53. https://doi.org/10.1186/s13045-017-0423-1
Maude SL, Laetsch TW, Buechner J et al (2018) Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 378(5):439–448. https://doi.org/10.1056/NEJMoa1709866
Majzner RG, Mackall CL (2019) Clinical lessons learned from the first leg of the CAR T cell journey. Nat Med 25(9):1341–1355. https://doi.org/10.1038/s41591-019-0564-6
Roddie C, O’Reilly M, Dias Alves Pinto J et al (2019) Manufacturing chimeric antigen receptor T cells: Issues and challenges. Cytotherapy 21(3):327–340. https://doi.org/10.1016/j.jcyt.2018.11.009
Brudno JN, Kochenderfer JN (2019) Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev 34:45–55. https://doi.org/10.1016/j.blre.2018.11.002
Qasim W, Zhan H, Samarasinghe S et al (2017) Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med 9(374). https://doi.org/10.1126/scitranslmed.aaj2013
Ho JY, Wang L, Liu Y et al (2021) Promoter usage regulating the surface density of CAR molecules may modulate the kinetics of CAR-T cells in vivo. Mol Ther - Methods Clin Dev 21:237–246. https://doi.org/10.1016/j.omtm.2021.03.007
Maloney DG, Grillo-López AJ, White CA et al (1997) IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90(6):2188–2195
Funding
This work was supported by grants from the General Project of National Natural Science Foundation of China (81970180), and the Key Science and Technology Bureau (20YFZCSY00800), as well as Tianjin Key Medical Discipline (Specialty) Construction Project.
Author information
Authors and Affiliations
Contributions
MFZ designed the research. XX, XJ, YBY, DNX, RS, YXW, and WYL performed the research. XX, XJ, YBY, and RS analyzed the data. XX, XJ, and YBY wrote the manuscript. XX, XJ, YBY, and MFZ revised the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Tianjin First Central Hospital. The patients/participants provided their written informed consent to participate in this study. The animal study was reviewed and approved by Ethics Committee of Tianjin First Central Hospital.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiong, X., Yu, Y., Jin, X. et al. Functional Validation of the RQR8 Suicide /Marker Gene in CD19 CAR-T Cells and CLL1CAR-T Cells. Ann Hematol 102, 1523–1535 (2023). https://doi.org/10.1007/s00277-023-05227-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05227-0