Abstract
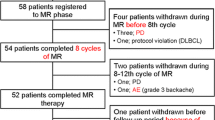
The evidence for the safety and efficacy of adding rituximab to intensive chemotherapy in pediatric patients with aggressive mature B cell non-Hodgkin lymphoma/leukemia (B-NHL/B-AL) is not yet robust. In this prospective multi-institutional trial, 419 evaluable patients ≤ 16 years of age with newly diagnosed B-NHL/B-AL were enrolled. Patients were stratified into 4 risk groups according to stage, resection status, and serum lactate dehydrogenase. Patients in group R1 received 3 therapy courses in the treatment order A-B-A. Patients in group R2 received 5 courses A-B-A-B-A. Patients in group R3 received 6 courses A-BB-AA-BB-AA-BB. For patients in group R4, rituximab was added to the chemotherapy backbone for patients in R3 (A-RBB-RAA-RBB-RAA-BB). At a median follow-up of 54 months, the 4-year event-free survival (EFS) for the entire group was 88.3 ± 1.6% (76.0 ± 4.3% in the historical study). The EFS rates according to the intention-to-treat principle were 100%, 98.6 ± 1.2%, 94.2 ± 1.8%, and 73.5 ± 3.7% for patients in treatment groups R1, R2, R3, and R4, respectively (P < 0.001). There were 9 (2.1%) toxic deaths due to infection during treatment. Regarding the toxicities of rituximab, grade 3/4 thrombocytopenia, mucositis, and infection occurred in 44.0%, 33.3%, and 64.0% after courses R-BB and grade 3/4 neutropenia, thrombocytopenia, and infection occurred in 96.3%, 77.8%, and 54.1% after courses RAA. The addition of rituximab to intensive chemotherapy is feasible even in a developing country. EFS was significantly improved when compared with the historical data. clinicals.gov identifier: NCT02405676.



Similar content being viewed by others
References
Cairo MS (2019) Rituximab in the treatment of childhood mature B-cell lymphoma: “where do we go from here.” Br J Haematol 185:1017–1020
Magrath I, Adde M, Sha A et al (1996) Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J CLinn Oncol 14:925–934
Tang JY, Pan C, Chen J et al (2002) Outcome of B-cell non-Hodgkin lymphoma protocol CCCG-B NHL97: a report from Chinese multi-center cooperative group. Med Pediatr Oncol 39:212–214
Subspecialty Group of Hematology the Society of Pediatrics Chinese Medical Association; Committee of Pediatrics Chinese Anti-Cancer Association (2014) A collaborative study of children with mature B-cell non-Hodgkin’s lymphoma in China]. [Article in Chinese. Zhonghua Er Ke Za Zhi 52:649–654
Woessmann W, Seidemann K, Mann G et al (2005) The impact of the methotrexate administration schedule and dose in the treatment of children and adolescents with B-cell neoplasms: a report of the BFM Group Study NHL-BFM95. Blood 105:948–968
Reiter A, Schrappe M, Tiemann M et al (1999) Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: a Report of the Berlin-Frankfurt-Müster Group Trial NHL-BFM 90. Blood 94:3294–3306
Swerdlow SH, Campo E, Harris NL, et al (2008) WHO classification of tumors of haematopoietic and lymphoid tissues (ed 4th) Lyon France IARC Press
Rosolen A, Perkins SL, Pinkerton CR et al (2015) Revised international pediatric non-Hodgkin lymphoma staging system. J Clin Oncol 33:2112–2118
Cairo MS, Gerrard M, Sposto R et al (2007) Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood 109:2736–2743
Minard-Colin V, Aupérin A, Pillon M et al (2020) Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med 382:2207–2219
Riad R, Omar W, Sidhom I et al (2010) False-positive F-18 FDG uptake in PET/CT studies in pediatric patients with abdominal Burkitt’s lymphoma. Nucl Med Commun 31:232–238
Pinkerton CR, Hann I, Eden OB, Gerrard M, Berry J, Mott MG (1991) Outcome in stage III non-Hodgkin’s lymphoma in children (UKCCSG study NHL 86) – how much treatment is needed? United Kingdom Children’s Cancer Study Group. Br J Cancer 64:583–587
Funding
This study was supported by the National Key Clinical Specialty Project.
Author information
Authors and Affiliations
Contributions
YJG and JYT designed and planned the study. YJG wrote the first draft of the manuscript. YLH analyzed the data and prepared the survival curve figure. JG involved in critical reviewing. YJG, YJF, JG, LCY, AGL, XLJ, JL, and JYT contributed to patient enrollment. YLH, JW, LCY, MX, XG, and JL involved in data collection. All authors decided to publish the paper and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gao, YJ., Fang, YJ., Gao, J. et al. A prospective multicenter study investigating rituximab combined with intensive chemotherapy in newly diagnosed pediatric patients with aggressive mature B cell non-Hodgkin lymphoma (CCCG-BNHL-2015): a report from the Chinese Children’s Cancer Group. Ann Hematol 101, 2035–2043 (2022). https://doi.org/10.1007/s00277-022-04904-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04904-w