Abstract
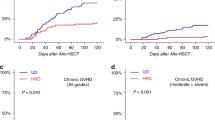
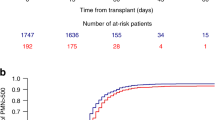
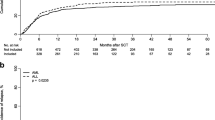
Allogeneic hematopoietic cell transplantation (HCT) is a potentially curative therapy for several malignant hematologic diseases and alternative donors, including haploidentical, play a significant role in HCT. Despite the increasing use of haplo-HCT with PTCy, some questions remain open. The objective of the present study was to investigate risk factors for adverse outcomes after haplo-HCT with PTCy. This is a retrospective study conducted at two Brazilian centers. A total of 103 patients with hematologic malignancies who underwent first allogeneic, haploidentical HCT with PTCy were included. Risk factors for death were age at transplant (HR = 1.03 for each year; p = 0.002) and high/very high disease risk index (DRI; HR = 2.77; p = 0.0007) and mother as the donor compared with other donors (HR = 3.53; p = 0.005). In multivariate analysis, PFS was significantly poorer for older patients (HR = 1.02; p = 0.006), high/very high DRI (HR = 2.39; p = 0.003), and mother as the donor compared with other donors (HR = 3.18; p = 0.006). Relapse rate was higher for high/very high DRI (HR = 4.01; p = 0.002) and mother as the donor compared with other donors (HR = 2.52; p = 0.05). NRM was higher for older patients (HR = 1.03 for each year; p = 0.03). Tacrolimus was a protective factor for grades II–IV aGVHD (HR = 0.46; p = 0.04) compared with cyclosporine. Peripheral blood (PBSC) was a risk factor for cGVHD (HR = 3.48; p = 0.006), while tacrolimus was protective (HR = 0.30; p = 0.009). Mother as the donor compared with other donors was also a risk factor for poorer OS, PFS, and relapse, suggesting that this combination should be avoided. Tacrolimus was protective for both grades II–IV aGVHD and cGVHD, suggesting that tacrolimus may be more effective than cyclosporine in preventing GVHD. PBSC was a risk factor for cGVHD without any impact on relapse. Prospective studies comparing tacrolimus with cyclosporine are awaited.




Similar content being viewed by others
References
Raiola AM, Dominietto A, di Grazia C et al (2014) Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 20:1573–1579. https://doi.org/10.1016/j.bbmt.2014.05.029
O’Reilly RJ, Keever C, Kernan NA et al (1987) HLA nonidentical T cell depleted marrow transplants: a comparison of results in patients treated for leukemia and severe combined immunodeficiency disease. Transplant Proc 19:55–60
Aversa F, Tabilio A, Terenzi A et al (1994) Successful engraftment of T-cell-depleted haploidentical “three-loci” incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood 84:3948–3955
Bethge WA, Faul C, Bornhäuser M et al (2008) Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis 40:13–19. https://doi.org/10.1016/j.bcmd.2007.07.001
Rizzieri DA, Koh LP, Long GD et al (2007) Partially matched, nonmyeloablative allogeneic transplantation: clinical outcomes and immune reconstitution. J Clin Oncol 25:690–697. https://doi.org/10.1200/JCO.2006.07.0953
Luznik L, O’Donnell PV, Symons HJ et al (2008) HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant 14:641–650. https://doi.org/10.1016/j.bbmt.2008.03.005
Arcuri LJ, Hamerschlak N, Rocha V et al (2021) Outcomes after haploidentical hematopoietic cell transplantation with post-transplantation cyclophosphamide: a systematic review and meta-analysis comparing myeloablative with reduced-intensity conditioning regimens and bone marrow with peripheral blood stem cell grafts. Transplant Cell Ther S2666636721009969. https://doi.org/10.1016/j.jtct.2021.06.011
Wang Y, Chang Y-J, Xu L-P et al (2014) Who is the best donor for a related HLA haplotype-mismatched transplant? Blood 124:843–850. https://doi.org/10.1182/blood-2014-03-563130
Fuchs EJ (2012) Haploidentical transplantation for hematologic malignancies: where do we stand? Hematology Am Soc Hematol Educ Program 2012:230–236. https://doi.org/10.1182/asheducation-2012.1.230
Ciurea SO, Al Malki MM, Kongtim P et al (2019) The European Society for Blood and Marrow Transplantation (EBMT) consensus recommendations for donor selection in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant. https://doi.org/10.1038/s41409-019-0499-z
Huselton E, Slade M, Trinkaus KM et al (2018) Propensity score analysis of conditioning intensity in peripheral blood haploidentical hematopoietic cell transplantation. Biol Blood Marrow Transplant 24:2047–2055. https://doi.org/10.1016/j.bbmt.2018.05.024
Bashey A, Zhang M-J, McCurdy SR et al (2017) Mobilized peripheral blood stem cells versus unstimulated bone marrow as a graft source for T-cell-replete haploidentical donor transplantation using post-transplant cyclophosphamide. J Clin Oncol 35:3002–3009. https://doi.org/10.1200/JCO.2017.72.8428
Armand P, Gibson CJ, Cutler C et al (2012) A disease risk index for patients undergoing allogeneic stem cell transplantation. Blood 120:905–913. https://doi.org/10.1182/blood-2012-03-418202
Harris AC, Young R, Devine S et al (2016) International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 22:4–10. https://doi.org/10.1016/j.bbmt.2015.09.001
Jagasia MH, Greinix HT, Arora M et al (2015) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21:389-401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001
Solomon SR, Aubrey MT, Zhang X et al (2018) Selecting the best donor for haploidentical transplant: impact of HLA, killer cell immunoglobulin-like receptor genotyping, and other clinical variables. Biol Blood Marrow Transplant 24:789–798. https://doi.org/10.1016/j.bbmt.2018.01.013
DeZern AE, Franklin C, Tsai H-L et al (2021) Relationship of donor age and relationship to outcomes of haploidentical transplantation with posttransplant cyclophosphamide. Blood Adv 5:1360–1368. https://doi.org/10.1182/bloodadvances.2020003922
McCurdy SR, Zhang M-J, St Martin A et al (2018) Effect of donor characteristics on haploidentical transplantation with posttransplantation cyclophosphamide. Blood Adv 2:299–307. https://doi.org/10.1182/bloodadvances.2017014829
Wang Y, Liu Q-F, Lin R et al (2021) Optimizing antithymocyte globulin dosing in haploidentical hematopoietic cell transplantation: long-term follow-up of a multicenter, randomized controlled trial. Science Bulletin 66:2498–2505. https://doi.org/10.1016/j.scib.2021.06.002
Stern M, Ruggeri L, Mancusi A et al (2008) Survival after T cell-depleted haploidentical stem cell transplantation is improved using the mother as donor. Blood 112:2990–2995. https://doi.org/10.1182/blood-2008-01-135285
Ruggeri A, Labopin M, Bacigalupo A et al (2018) Bone marrow versus mobilized peripheral blood stem cells in haploidentical transplants using posttransplantation cyclophosphamide. Cancer 124:1428–1437. https://doi.org/10.1002/cncr.31228
Cutler C, Giri S, Jeyapalan S et al (2001) Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol 19:3685–3691. https://doi.org/10.1200/JCO.2001.19.16.3685
Eapen M, Logan BR, Confer DL et al (2007) Peripheral blood grafts from unrelated donors are associated with increased acute and chronic graft-versus-host disease without improved survival. Biol Blood Marrow Transplant 13:1461–1468. https://doi.org/10.1016/j.bbmt.2007.08.006
Körbling M, Anderlini P (2001) Peripheral blood stem cell versus bone marrow allotransplantation: does the source of hematopoietic stem cells matter? Blood 98:2900–2908. https://doi.org/10.1182/blood.v98.10.2900
Flowers MED, Inamoto Y, Carpenter PA et al (2011) Comparative analysis of risk factors for acute graft-versus-host disease and for chronic graft-versus-host disease according to National Institutes of Health consensus criteria. Blood 117:3214–3219. https://doi.org/10.1182/blood-2010-08-302109
Hahn T, McCarthy PL, Zhang M-J et al (2008) Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. J Clin Oncol 26:5728–5734. https://doi.org/10.1200/JCO.2008.17.6545
Flowers MED, Parker PM, Johnston LJ et al (2002) Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood 100:415–419. https://doi.org/10.1182/blood-2002-01-0011
Chiusolo P, Bug G, Olivieri A et al (2018) A modified post-transplant cyclophosphamide regimen, for unmanipulated haploidentical marrow transplantation, in acute myeloid leukemia: a multicenter study. Biol Blood Marrow Transplant 24:1243–1249. https://doi.org/10.1016/j.bbmt.2018.01.031
Castagna L, Bramanti S, Furst S et al (2016) Tacrolimus compared with cyclosporine A after haploidentical T-cell replete transplantation with post-infusion cyclophosphamide. Bone Marrow Transplant 51:462–465. https://doi.org/10.1038/bmt.2015.289
Inamoto Y, Flowers MED, Appelbaum FR et al (2011) A retrospective comparison of tacrolimus versus cyclosporine with methotrexate for immunosuppression after allogeneic hematopoietic cell transplantation with mobilized blood cells. Biol Blood Marrow Transplant 17:1088–1092. https://doi.org/10.1016/j.bbmt.2011.01.017
Kanda Y, Kobayashi T, Mori T et al (2016) A randomized controlled trial of cyclosporine and tacrolimus with strict control of blood concentrations after unrelated bone marrow transplantation. Bone Marrow Transplant 51:103–109. https://doi.org/10.1038/bmt.2015.222
Nash RA, Antin JH, Karanes C et al (2000) Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 96:2062–2068
Ratanatharathorn V, Nash RA, Przepiorka D et al (1998) Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood 92:2303–2314
Hiraoka A, Ohashi Y, Okamoto S et al (2001) Phase III study comparing tacrolimus (FK506) with cyclosporine for graft-versus-host disease prophylaxis after allogeneic bone marrow transplantation. Bone Marrow Transplant 28:181–185. https://doi.org/10.1038/sj.bmt.1703097
McCurdy SR, Kanakry JA, Showel MM et al (2015) Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 125:3024–3031. https://doi.org/10.1182/blood-2015-01-623991
Ciurea SO, Rodrigues M, Giralt S, de Lima M (2009) Aging, acute myelogenous leukemia, and allogeneic transplantation: do they belong in the same sentence? Clin Lymphoma Myeloma 9:289–297. https://doi.org/10.3816/CLM.2009.n.057
Gratwohl A (2012) The EBMT risk score. Bone Marrow Transplant 47:749–756. https://doi.org/10.1038/bmt.2011.110
Ciurea SO, Shah MV, Saliba RM et al (2018) Haploidentical transplantation for older patients with acute myeloid leukemia and myelodysplastic syndrome. Biol Blood Marrow Transplant 24:1232–1236. https://doi.org/10.1016/j.bbmt.2017.09.005
Souza MA, Passos AM, Treitinger A, Spada C (2010) Seroprevalence of cytomegalovirus antibodies in blood donors in southern, Brazil. Rev Soc Bras Med Trop 43:359–361
Solomon SR, Solh M, Morris LE et al (2016) Myeloablative conditioning with PBSC grafts for T cell-replete haploidentical donor transplantation using posttransplant cyclophosphamide. Adv Hematol 2016:9736564. https://doi.org/10.1155/2016/9736564
Author information
Authors and Affiliations
Contributions
VJTL, AFS, LNK, MNK, AAFR, CCS, NH, and LJA were responsible for the study design and local conduction of the study. DL and MC were responsible for local conduction of the study. VJTL, CCS, and LJA collected the data. VJTL, LNK, NH, and LJA wrote and critically reviewed the manuscript.
Corresponding author
Ethics declarations
Consent to participate
Ethics committee waived informed consent.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors declare that the manuscript is original and has not been published before.
Rights and permissions
About this article
Cite this article
de Lima, V.J.T., da Silva, A.F., Kerbauy, L.N. et al. Risk factors for adverse outcomes following haploidentical hematopoietic cell transplantation with posttransplant cyclophosphamide: a two-center analysis. Ann Hematol 101, 1795–1802 (2022). https://doi.org/10.1007/s00277-022-04865-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-022-04865-0