Abstract
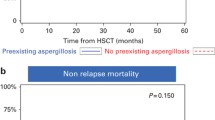
There is a matter of debate about the clinical impact of cytomegalovirus (CMV) reactivation on the development of late-onset invasive aspergillosis (IA), which occurs 40 days or later after allogeneic hematopoietic stem cell transplantation (HSCT). Using a Japanese transplant registry database, we analyzed the risk factors for the development of late-onset IA in 21,015 patients who underwent their first allogeneic HSCT between 2006 and 2017. CMV reactivation was defined as the initiation of preemptive anti-CMV antiviral therapy. Overall, there were 582 cases of late-onset IA, which occurred at a median of 95 days after HSCT. The 2-year cumulative incidence was 3.4% (95% confidence interval (CI), 3.0–3.9) in patients with CMV reactivation within 40 days after HSCT and 2.5% (95% CI, 2.3–2.8) in those without it (P < 0.001). In a multivariate analysis, CMV reactivation as a time-dependent covariate was significantly associated with the development of late-onset IA (hazard ratio (HR) 1.40, P < 0.001), as well as grade II–IV acute GVHD, age > 50 and HCT-CI ≥ 3 in the entire cohort. If we focus on the subgroup without grade II–IV acute GVHD, which is generally an indication for systemic corticosteroid therapy (n = 12,622), CMV reactivation was still a significant factor for the development of late-onset IA (HR 1.37, P = 0.045) as well as age > 50 years, HCT-CI ≥ 3, and cord blood transplantation. In conclusion, CMV reactivation was associated with an increased risk of late-onset IA after allogeneic HSCT independently of acute GVHD. Close monitoring for late-onset IA is necessary for patients who develop CMV reactivation even without grade II–IV acute GVHD.

Similar content being viewed by others
References
Neofytos D, Horn D, Anaissie E, Steinbach W, Olyaei A, Fishman J, Pfaller M, Chang C, Webster K, Marr K (2009) Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin Infect Dis 48(3):265–273. https://doi.org/10.1086/595846
Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG (2010) Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 50(8):1091–1100. https://doi.org/10.1086/651263
Marr KA, Carter RA, Boeckh M, Martin P, Corey L (2002) Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood 100(13):4358–4366. https://doi.org/10.1182/blood-2002-05-1496
Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, Martin PJ, Storb RF, Marr KA (2003) Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood 102(3):827–833. https://doi.org/10.1182/blood-2003-02-0456
Garcia-Vidal C, Upton A, Kirby KA, Marr KA (2008) Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation. Clin Infect Dis 47(8):1041–1050. https://doi.org/10.1086/591969
Mikulska M, Raiola AM, Bruno B, Furfaro E, Van Lint MT, Bregante S, Ibatici A, Del Bono V, Bacigalupo A, Viscoli C (2009) Risk factors for invasive aspergillosis and related mortality in recipients of allogeneic SCT from alternative donors: an analysis of 306 patients. Bone Marrow Transplant 44(6):361–370. https://doi.org/10.1038/bmt.2009.39
Li L, Wang J, Zhang W, Yang J, Chen L, Lv S (2012) Risk factors for invasive mold infections following allogeneic hematopoietic stem cell transplantation: a single center study of 190 recipients. Scand J Infect Dis 44(2):100–107. https://doi.org/10.3109/00365548.2011.623311
Kimura SI, Takeshita J, Kawamura M, Kawamura S, Yoshino N, Misaki Y, Yoshimura K, Matsumi S, Gomyo A, Akahoshi Y, Tamaki M, Kusuda M, Kameda K, Wada H, Kawamura K, Sato M, Terasako-Saito K, Tanihara A, Nakasone H, Kako S, Kanda Y (2020) Association between the kinetics of cytomegalovirus reactivation evaluated in terms of the area under the curve of cytomegalovirus antigenemia and invasive mold infection during the post-engraftment phase after allogeneic hematopoietic stem cell transplantation. Transpl Infect Dis:e13387. doi:https://doi.org/10.1111/tid.13387
Freeman RB Jr (2009) The “indirect” effects of cytomegalovirus infection. Am J Transplant 9(11):2453–2458. https://doi.org/10.1111/j.1600-6143.2009.02824.x
Frascaroli G, Varani S, Blankenhorn N, Pretsch R, Bacher M, Leng L, Bucala R, Landini MP, Mertens T (2009) Human cytomegalovirus paralyzes macrophage motility through down-regulation of chemokine receptors, reorganization of the cytoskeleton, and release of macrophage migration inhibitory factor. J Immunol 182(1):477–488. https://doi.org/10.4049/jimmunol.182.1.477
Ljungman P, Perez-Bercoff L, Jonsson J, Avetisyan G, Sparrelid E, Aschan J, Barkholt L, Larsson K, Winiarski J, Yun Z, Ringden O (2006) Risk factors for the development of cytomegalovirus disease after allogeneic stem cell transplantation. Haematologica 91(1):78–83
Atsuta Y, Suzuki R, Yoshimi A, Gondo H, Tanaka J, Hiraoka A, Kato K, Tabuchi K, Tsuchida M, Morishima Y, Mitamura M, Kawa K, Kato S, Nagamura T, Takanashi M, Kodera Y (2007) Unification of hematopoietic stem cell transplantation registries in Japan and establishment of the TRUMP system. Int J Hematol 86(3):269–274. https://doi.org/10.1532/IJH97.06239
Atsuta Y (2016) Introduction of Transplant Registry Unified Management Program 2 (TRUMP2): scripts for TRUMP data analyses, part I (variables other than HLA-related data). Int J Hematol 103(1):3–10. https://doi.org/10.1007/s12185-015-1894-x
Kanda J (2016) Scripts for TRUMP data analyses. Part II (HLA-related data): statistical analyses specific for hematopoietic stem cell transplantation. Int J Hematol 103 (1):11–19. doi:https://doi.org/10.1007/s12185-015-1907-9
De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW, Patterson TF, Maschmeyer G, Bille J, Dismukes WE, Herbrecht R, Hope WW, Kibbler CC, Kullberg BJ, Marr KA, Munoz P, Odds FC, Perfect JR, Restrepo A, Ruhnke M, Segal BH, Sobel JD, Sorrell TC, Viscoli C, Wingard JR, Zaoutis T, Bennett JE (2008) Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 46(12):1813–1821. https://doi.org/10.1086/588660
Takenaka K, Nishida T, Asano-Mori Y, Oshima K, Ohashi K, Mori T, Kanamori H, Miyamura K, Kato C, Kobayashi N, Uchida N, Nakamae H, Ichinohe T, Morishima Y, Suzuki R, Yamaguchi T, Fukuda T (2015) Cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation is associated with a reduced risk of relapse in patients with acute myeloid leukemia who survived to day 100 after transplantation: the Japan Society for Hematopoietic Cell Transplantation Transplantation-Related Complication Working Group. Biol Blood Marrow Transplant 21(11):2008–2016. https://doi.org/10.1016/j.bbmt.2015.07.019
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15 (6):825–828
Shulman HM, Sale GE, Lerner KG, Barker EA, Weiden PL, Sullivan K, Gallucci B, Thomas ED, Storb R (1978) Chronic cutaneous graft-versus-host disease in man. Am J Pathol 91(3):545–570
Sullivan KM, Shulman HM, Storb R, Weiden PL, Witherspoon RP, McDonald GB, Schubert MM, Atkinson K, Thomas ED (1981) Chronic graft-versus-host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 57(2):267–276
Busca A, Passera R, Maffini E, Festuccia M, Brunello L, Dellacasa CM, Aydin S, Frairia C, Manetta S, Butera S, Iovino G, Giaccone L, Sorror M, Storb R, De Rosa FG, Bruno B (2018) Hematopoietic cell transplantation comorbidity index and risk of developing invasive fungal infections after allografting. Bone Marrow Transplant 53(10):1304–1310. https://doi.org/10.1038/s41409-018-0161-1
Kanda Y (2013) Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant 48(3):452–458. https://doi.org/10.1038/bmt.2012.244
Yong MK, Slavin MA, Kontoyiannis DP (2018) Invasive fungal disease and cytomegalovirus infection: is there an association? Curr Opin Infect Dis 31(6):481–489. https://doi.org/10.1097/QCO.0000000000000502
Choi JK, Cho SY, Yoon SS, Moon JH, Kim SH, Lee JH, Kim JS, Cheong JW, Jang JH, Seo BJ, Kim YJ, Lee HJ, Lee J, Lee JW, Lee DG (2017) Epidemiology and risk factors for invasive fungal diseases among allogeneic hematopoietic stem cell transplant recipients in Korea: results of “RISK” study. Biol Blood Marrow Transplant 23(10):1773–1779. https://doi.org/10.1016/j.bbmt.2017.06.012
Yong MK, Ananda-Rajah M, Cameron PU, Morrissey CO, Spencer A, Ritchie D, Cheng AC, Lewin SR, Slavin M (2017) Cytomegalovirus reactivation is associated with increased risk of late-onset invasive fungal disease after allogeneic hematopoietic stem cell transplantation: a multicenter study in the current era of viral load monitoring. Biol Blood Marrow Transplant 23(11):1961–1967. https://doi.org/10.1016/j.bbmt.2017.07.025
Montesinos P, Rodriguez-Veiga R, Boluda B, Martinez-Cuadron D, Cano I, Lancharro A, Sanz J, Arilla MJ, Lopez-Chulia F, Navarro I, Lorenzo I, Salavert M, Peman J, Calvillo P, Martinez J, Carpio N, Jarque I, Sanz GF, Sanz MA (2015) Incidence and risk factors of post-engraftment invasive fungal disease in adult allogeneic hematopoietic stem cell transplant recipients receiving oral azoles prophylaxis. Bone Marrow Transplant 50(11):1465–1472. https://doi.org/10.1038/bmt.2015.181
Wald A, Leisenring W, van Burik JA, Bowden RA (1997) Epidemiology of Aspergillus infections in a large cohort of patients undergoing bone marrow transplantation. J Infect Dis 175(6):1459–1466. https://doi.org/10.1086/516480
Servais S, Lengline E, Porcher R, Carmagnat M, Peffault de Latour R, Robin M, Sicre de Fontebrune F, Clave E, Maki G, Granier C, Xhaard A, Dhedin N, Molina JM, Toubert A, Moins-Teisserenc H, Socie G (2014) Long-term immune reconstitution and infection burden after mismatched hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 20(4):507–517. https://doi.org/10.1016/j.bbmt.2014.01.001
Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, Clancy CJ, Wingard JR, Lockhart SR, Groll AH, Sorrell TC, Bassetti M, Akan H, Alexander BD, Andes D, Azoulay E, Bialek R, Bradsher RW, Bretagne S, Calandra T, Caliendo AM, Castagnola E, Cruciani M, Cuenca-Estrella M, Decker CF, Desai SR, Fisher B, Harrison T, Heussel CP, Jensen HE, Kibbler CC, Kontoyiannis DP, Kullberg BJ, Lagrou K, Lamoth F, Lehrnbecher T, Loeffler J, Lortholary O, Maertens J, Marchetti O, Marr KA, Masur H, Meis JF, Morrisey CO, Nucci M, Ostrosky-Zeichner L, Pagano L, Patterson TF, Perfect JR, Racil Z, Roilides E, Ruhnke M, Prokop CS, Shoham S, Slavin MA, Stevens DA, Thompson GR, Vazquez JA, Viscoli C, Walsh TJ, Warris A, Wheat LJ, White PL, Zaoutis TE, Pappas PG (2020) Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis 71(6):1367–1376. https://doi.org/10.1093/cid/ciz1008
Arvanitis M, Anagnostou T, Mylonakis E (2015) Galactomannan and polymerase chain reaction-based screening for invasive aspergillosis among high-risk hematology patients: a diagnostic meta-analysis. Clin Infect Dis 61(8):1263–1272. https://doi.org/10.1093/cid/civ555
Maertens JA, Girmenia C, Bruggemann RJ, Duarte RF, Kibbler CC, Ljungman P, Racil Z, Ribaud P, Slavin MA, Cornely OA, Peter Donnelly J, Cordonnier C, European Conference on Infections in Leukaemia ajvotEGfB, Marrow Transplantation tEOfR, Treatment of Cancer tIHSa, European Conference on Infections in Leukaemia ajvotEGfB, Marrow Transplantation tEOfR, Treatment of Cancer tIHS, the European L (2018) European guidelines for primary antifungal prophylaxis in adult haematology patients: summary of the updated recommendations from the European Conference on Infections in Leukaemia. J Antimicrob Chemother 73 (12):3221–3230. doi:https://doi.org/10.1093/jac/dky286
Ljungman P, de la Camara R, Robin C, Crocchiolo R, Einsele H, Hill JA, Hubacek P, Navarro D, Cordonnier C, Ward KN, European Conference on Infections in Leukaemia g (2019) Guidelines for the management of cytomegalovirus infection in patients with haematological malignancies and after stem cell transplantation from the 2017 European Conference on Infections in Leukaemia (ECIL 7). Lancet Infect Dis 19 (8):e260-e272. https://doi.org/10.1016/S1473-3099(19)30107-0
Acknowledgements
The authors are grateful for the work of all of the physicians and data managers at the centers that contributed valuable data on transplantation to the JSTCT. We would also like to thank all of the members of the Transplant Registry Unified Management committees at JSTCT for their dedicated data management.
Author information
Authors and Affiliations
Contributions
SI.K. designed the study, analyzed the data, and wrote the manuscript. M. Tamaki, K.O., and S. Seo advised on methods and revised the manuscript. N.U., A.I, Y.O., K.I., T.E., M. Tanaka, S. Shiratori, H. Nakamae, M.S., and T.K. collected the data and revised the manuscript. M.O., T.F., and Y.K. collected the data, revised the manuscript, and were responsible for data management at JSTCT. Y.A. managed the unified registry database and revised the manuscript. H. Nakasone designed the study, advised on the methods, wrote the manuscript, and was responsible for the project of the JSTCT Transplant Complications Working Group.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the data management committee of the Japanese Society for Transplantation and Cellular Therapy and by the institutional review board of Jichi Medical University Saitama Medical Center (Saitama, Japan). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The Transplant Registry Unified Management Program (TRUMP) database of the Japanese Society for Transplantation and Cellular Therapy and the Japanese Data Center for Hematopoietic Cell Transplantation includes physician-reviewed data. Observational studies based on the TRUMP database are performed with informed consent.
Conflict of interest
Shun-ichi Kimura has received a grant from the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP20K17406 and received personal fees from Asahi Kasei, Sumitomo Dainippon Pharma, MSD, Astellas, Pfizer, Kyowa Kirin, Chugai Pharmaceutical Co., Ltd., Bristol Myers Squibb, Celgene, Ono Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Nippon Kayaku. Sachiko Seo has received personal fees from Janssen Pharmaceuticals. K.K. Hirohisa Nakamae has received honoraria from Amgen Astellas BioPharma K.K., Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Kyowa Hakko Kirin Co., Ltd, Celgene Corporation, Daiichi Sankyo Company, Limited, Takeda Pharmaceutical Company Limited, Chugai Pharmaceutical Co., Ltd., Japan Blood Products Organization, Nippon Shinyaku Co., Ltd., Novartis, Pfizer Japan Inc., Bristol Myers Squibb, Shire Japan KK, and Ono Pharmaceutical Co., Ltd. and received grants from Astellas Pharma Inc., Otsuka Pharmaceutical Co., Ltd., Novartis, Bristol Myers Squibb, and PPD-SNBL K.K. Masashi Sawa has received personal fees from Chugai, Pfizer, Astellas, Nippon-Shinyaku, Ono, MSD, Bristol Myers Squibb, Kyowa Hakko Kirin, Asahi Kasei, Novartis, Eisai, Otsuka, Sumitomo Dainippon, Sanofi, Takeda, Celgene, Mochida, Shire, and Mundipharma. Yoshinobu Kanda has received honoraria from Merck Sharp & Dohme, Astellas, Sumitomo Dainippon Pharma, Chugai Pharmaceutical Co., Ltd., and Pfizer, and received research funding from Sumitomo Dainippon Pharma, Eisai Co., Ltd., Chugai Pharmaceutical Co., Ltd., Astellas, Kyowa Kirin, Takeda Pharmaceutical Company Limited., Ono Pharmaceutical Co., Ltd., and Shionogi & Co., Ltd. Hideki Nakasone has received grants from Japan Agency for Medical Research and Development and Japan Society for the Promotion of Science, and received personal fees from Takeda Pharmaceutical, Otsuka Pharmaceutical, Bristol Myers Squibb, Celgene, Pfizer, Novartis, Janssen Pharmaceutical K.K., Eisai, Chugai Pharmaceutical, and Nippon Shinyaku. Other authors: none to declare.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, Si., Tamaki, M., Okinaka, K. et al. Cytomegalovirus reactivation is associated with an increased risk of late-onset invasive aspergillosis independently of grade II–IV acute graft-versus-host disease in allogeneic hematopoietic stem cell transplantation: JSTCT Transplant Complications Working Group. Ann Hematol 100, 3029–3038 (2021). https://doi.org/10.1007/s00277-021-04660-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04660-3