Abstract
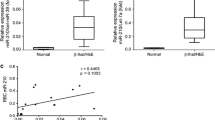
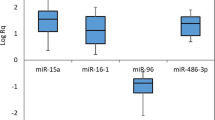
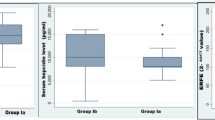
Tissue iron overload is a life-threatening scenario in children with transfusion-dependent β-thalassemia major, miRNAs that are involved in iron hemostasis could serve as therapeutic targets for control of iron overload. We aimed to find out the association between three iron-related miRNAs “miR-let-7d, miR-122, and miR-200b” and excess iron in tissues, in transfusion-dependent β-thalassemia major patients. Circulating miRNA expressions are measured in peripheral blood (PB) samples using qPCR of transfusion-dependent (TDT) β-thalassemia patients (n = 140) and normalized to non-transfusion-dependent (NTDT) β-thalassemia (n = 45). Results revealed that plasma expression levels of miR-let-7d and miR-200b were significantly downregulated in TDT patients; however, miR-122 was upregulated. In terms of tissue iron load, aberrant expression of miRNAs was significantly associated with increased—iron accumulation in hepatic and cardiac tissues. We concluded that circulating miRNAs are strong candidates that associate iron hemostasis in transfusion-dependent β-thalassemia major patients. And by extension, targeting miR-let-7d, miR-122, and miR-200 might serve as novel sensitive, specific and non-invasive predictor biomarkers for cellular damage under condition of tissue iron excess.


Similar content being viewed by others
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable.
Change history
06 October 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00277-021-04691-w
References
Alateeq S et al (2018) Identification of on-target mutagenesis during correction of a beta-thalassemia splice mutation in iPS cells with optimised CRISPR/Cas9-double nickase reveals potential safety concerns. APL Bioeng 2(4):046103
Cappellini MD, Cohen A, Porter J, Taher A, Viprakasit V (2014) Guidelines for the management of transfusion dependent thalassaemia (TDT), 3rd edn. Thalassaemia International Federation, Nicosia. https://pubmed.ncbi.nlm.nih.gov/25610943
Musallam KM, Rivella S, Vichinsky E, Rachmilewitz EA (2013) Non-transfusion-dependent thalassemias, (in eng). Haematologica 98(6):833–844
Taher AT, Saliba AN (2017) Iron overload in thalassemia: different organs at different rates, (in eng), Hematology. Am Soc Hematol Educ Program 2017(1):265–271
Zakaria M and Hassan T (2019) Oxidative stress and iron overload in β-thalassemia: An Overview, Beta Thalassemia, IntechOpen. https://doi.org/10.5772/intechopen.90492. https://www.intechopen.com/chapters/70487
Chaichompoo P et al (2019) Abnormal red blood cell morphological changes in thalassaemia associated with iron overload and oxidative stress. J Clin Pathol 72:jclinpath-2019
De Sanctis V, Giovannini M (2011) Endocrine histology findings in a prepubertal thalassemic girl with multiple endocrine complications secondary to iron overload, (in eng). Georgian Med News 193:51–55
Bajoria R, Chatterjee R (2011) Hypogonadotrophic hypogonadism and diminished gonadal reserve accounts for dysfunctional gametogenesis in thalassaemia patients with iron overload presenting with infertility, (in eng). Hemoglobin 35(5–6):636–642
Gattermann N, Rachmilewitz EA (2011) Iron overload in MDS-pathophysiology, diagnosis, and complications, (in eng). Ann Hematol 90(1):1–10
Farmaki K, Tzoumari I, Pappa C (2011) Oral chelators in transfusion-dependent thalassemia major patients may prevent or reverse iron overload complications, (in eng). Blood Cells Mol Dis 47(1):33–40
Liu Q, Sun L, Tan Y, Wang G, Lin X, Cai L (2009) Role of iron deficiency and overload in the pathogenesis of diabetes and diabetic complications, (in eng). Curr Med Chem 16(1):113–129
Brittenham GM et al (1994) Efficacy of deferoxamine in preventing complications of iron overload in patients with thalassemia major, (in eng). N Engl J Med 331(9):567–573
Telfer PT, Prestcott E, Holden S, Walker M, Hoffbrand AV, Wonke B (2000) Hepatic iron concentration combined with long-term monitoring of serum ferritin to predict complications of iron overload in thalassaemia major, (in eng). Br J Haematol 110(4):971–977
Pinto VM, Forni GL (2020) Management of iron overload in beta-thalassemia patients: clinical practice update based on case series. Int J Mol Sci 21(22):8771. https://doi.org/10.3390/ijms21228771
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function, (in eng). Cell 116(2):281–297
Miska EA (2005) How microRNAs control cell division, differentiation and death, (in eng). Curr Opin Genet Dev 15(5):563–568
Lytle JR, Yario TA, Steitz JA (2007) Target mRNAs are repressed as efficiently by microRNA-binding sites in the 5’ UTR as in the 3’ UTR, (in eng). Proc Natl Acad Sci USA 104(23):9667–9672
Ørom UA, Nielsen FC, Lund AH (2008) MicroRNA-10a binds the 5’UTR of ribosomal protein mRNAs and enhances their translation, (in eng). Mol Cell 30(4):460–471
Greene CM, Varley RB, Lawless MW (2013) MicroRNAs and liver cancer associated with iron overload: therapeutic targets unravelled, (in eng). World J Gastroenterol 19(32):5212–5226
Chen Y, Gelfond JA, McManus LM, Shireman PK (2009) Reproducibility of quantitative RT-PCR array in miRNA expression profiling and comparison with microarray analysis, (in eng). BMC Genomics 10:407
Reid G, Kirschner MB, van Zandwijk N (2011) Circulating microRNAs: association with disease and potential use as biomarkers, (in eng). Crit Rev Oncol Hematol 80(2):193–208
Mitchell PS et al (2008) Circulating microRNAs as stable blood-based markers for cancer detection, (in eng). Proc Natl Acad Sci USA 105(30):10513–10518
Masaki S, Ohtsuka R, Abe Y, Muta K, Umemura T (2007) Expression patterns of microRNAs 155 and 451 during normal human erythropoiesis, (in eng). Biochem Biophys Res Commun 364(3):509–514
Zhan M, Miller CP, Papayannopoulou T, Stamatoyannopoulos G, Song CZ (2007) MicroRNA expression dynamics during murine and human erythroid differentiation, (in eng). Exp Hematol 35(7):1015–1025
Leecharoenkiat K et al (2017) Plasma microRNA-451 as a novel hemolytic marker for β0-thalassemia/HbE disease, (in eng). Mol Med Rep 15(5):2495–2502
Sarakul O et al (2013) Enhanced erythroid cell differentiation in hypoxic condition is in part contributed by miR-210, (in eng). Blood Cells Mol Dis 51(2):98–103
Castoldi M, Muckenthaler MU (2012) Regulation of iron homeostasis by microRNAs, (in eng). Cell Mol Life Sci 69(23):3945–3952
Davis M, Clarke S (2013) Influence of microRNA on the maintenance of human iron metabolism, (in eng). Nutrients 5(7):2611–2628
Dykxhoorn DM et al (2009) miR-200 enhances mouse breast cancer cell colonization to form distant metastases, (in eng). PLoS One 4(9):e7181
Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T (2002) Identification of tissue-specific microRNAs from mouse. Curr Biol 12(9):735–739
Castoldi M et al (2011) The liver-specific microRNA miR-122 controls systemic iron homeostasis in mice, (in eng). J Clin Invest 121(4):1386–1396
Hou W, Tian Q, Steuerwald NM, Schrum LW, Bonkovsky HL (2012) The let-7 microRNA enhances heme oxygenase-1 by suppressing Bach1 and attenuates oxidant injury in human hepatocytes, (in eng). Biochim Biophys Acta 1819(11–12):1113–22
Chen X et al (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases, (in eng). Cell Res 18(10):997–1006
López P et al (2018) miR-155 and miR-122 expression of spermatozoa in obese subjects, (in eng). Front Genet 9:175–175
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs, (in eng). Genome Res 19(1):92–105
Yoshioka Y, Kosaka N, Ochiya T, Kato T (2012) Micromanaging iron homeostasis: hypoxia-inducible micro-RNA-210 suppresses iron homeostasis-related proteins, (in eng). J Biol Chem 287(41):34110–34119
Schaar DG, Medina DJ, Moore DF, Strair RK, Ting Y (2009) miR-320 targets transferrin receptor 1 (CD71) and inhibits cell proliferation, (in eng). Exp Hematol 37(2):245–255
Liao Y, Du X, Lönnerdal B (2010) miR-214 regulates lactoferrin expression and pro-apoptotic function in mammary epithelial cells, (in eng). J Nutr 140(9):1552–1556
Liao Y, Lönnerdal B (2010) miR-584 mediates post-transcriptional expression of lactoferrin receptor in Caco-2 cells and in mouse small intestine during the perinatal period, (in eng). Int J Biochem Cell Biol 42(8):1363–1369
Sangokoya C, Doss JF, Chi JT (2013) Iron-responsive miR-485–3p regulates cellular iron homeostasis by targeting ferroportin, (in eng). PLoS Genet 9(4):e1003408
Shpyleva SI et al (2011) Role of ferritin alterations in human breast cancer cells, (in eng). Breast Cancer Res Treat 126(1):63–71
Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ (2011) Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis, (in eng). Proc Natl Acad Sci USA 108(52):20970–20975
Hintze KJ, Katoh Y, Igarashi K, Theil EC (2007) Bach1 repression of ferritin and thioredoxin reductase1 is heme-sensitive in cells and in vitro and coordinates expression with heme oxygenase1, beta-globin, and NADP(H) quinone (oxido) reductase1, (in eng). J Biol Chem 282(47):34365–34371
Hou W, Tian Q, Zheng J, Bonkovsky HL (2010) MicroRNA-196 represses Bach1 protein and hepatitis C virus gene expression in human hepatoma cells expressing hepatitis C viral proteins, (in eng). Hepatology 51(5):1494–1504
Li Y et al (2012) Iron homeostasis regulates the activity of the microRNA pathway through poly(C)-binding protein 2. Cell Metab 15(6):895–904
Lis A, Paradkar PN, Singleton S, Kuo H-C, Garrick MD, Roth JA (2005) Hypoxia induces changes in expression of isoforms of the divalent metal transporter (DMT1) in rat pheochromocytoma (PC12) cells. Biochem Pharmacol 69(11):1647–1655
Andolfo I et al (2010) Regulation of divalent metal transporter 1 (DMT1) non-IRE isoform by the microRNA Let-7d in erythroid cells, (in eng). Haematologica 95(8):1244–1252
Arosio P, Levi S (2010) Cytosolic and mitochondrial ferritins in the regulation of cellular iron homeostasis and oxidative damage, (in eng). Biochim Biophys Acta 1800(8):783–792
Funding
The research is self-funded by the authors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by SAM & HF. The first draft of the manuscript was written by NE and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
The study protocol number FMASU 28115/2017 was approved by the research ethics committee of the Faculty of Medicine, Ain Shams University. All participants were informed of the objectives of the study and signed informed-consent forms.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: This article was originally published with an incorrect presentation of author affiliations.
Rights and permissions
About this article
Cite this article
El-Khazragy, N., Matbouly, S., Hanna, D.H. et al. Circulating miRNAs and tissue iron overload in transfusion-dependent β-thalassemia major: novel predictors and follow-up guide. Ann Hematol 100, 2909–2917 (2021). https://doi.org/10.1007/s00277-021-04639-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04639-0