Abstract
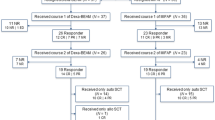
We investigated the feasibility and activity of an intensified dose-dense ABVD (dd-ABVD) regimen in patients with early-stage unfavorable Hodgkin lymphoma (HL). This prospective, multicenter, phase II study enrolled 96 patients with newly diagnosed, unfavorable stage I or II classical HL. The patients received four cycles of dd-ABVD followed by radiotherapy. Interim PET (PET-2) was mandatory after two courses. Primary endpoints were the evaluation of dd-ABVD feasibility and activity (incidence of PET-2 negativity). The feasibility endpoint was achieved with 48/52 (92.3%) patients receiving > 85% of the programmed dose. The mean dose intensity in the overall patient population (n = 96) was 93.7%, and the median duration of dd-ABVD was 85 days (range, 14–115) versus an expected duration of 84 days. PET-2 was available for 92/96 (95.8%) patients, of whom 79 were PET-2 negative (85.9%). In total, 90 (93.8%) patients showed complete response at the end of treatment. With a follow-up of 80.9 months (3.3–103.2), the median progression-free survival (PFS) and overall survival (OS) were not reached. At 84 months, PFS and OS rates were 88.4% and 95.7%, respectively. No evidence for a difference in PFS or OS was observed for PET-2-negative and PET-2-positive patients. Infections were documented in 8.3% and febrile neutropenia in 6.2% of cases. Four patients died: one had alveolitis at cycle 3, one death was unrelated to treatment, and two died from a secondary cancer. dd-ABVD is feasible and demonstrates activity in early-stage unfavorable HL. The predictive role of PET-2 positivity in early-stage unfavorable HL remains controversial. The study was registered in the EudraCT (reference number, 2011–003,191-36) and the ClinicalTrials.gov (reference number, NCT02247869) databases.



Similar content being viewed by others
Availability of data and materials
All data will be available from the corresponding author upon reasonable request.
Code availability
Not applicable.
References
Sasse S, Bröckelmann PJ, Goergen H, Plütschow A, Müller H, Kreissl S, Buerkle C, Borchmann S, Fuchs M, Borchmann P, Diehl V, Engert A (2017) Long-term follow-up of contemporary treatment in early-stage Hodgkin lymphoma: updated analyses of the German Hodgkin Study Group HD7, HD8, HD10, and HD11 trials. J Clin Oncol 35(18):1999–2007. https://doi.org/10.1200/JCO.2016.70.9410
Fermé C, Eghbali H, Meerwaldt JH, Rieux C, Bosq J, Berger F, Girinsky T, Brice P, van’t Veer MB, Walewski JA, Lederlin P, Tirelli U, Carde P, Van den Neste E, Gyan E, Monconduit M, Diviné M, Raemaekers JM, Salles G, Noordijk EM, Creemers GJ, Gabarre J, Hagenbeek A, Reman O, Blanc M, Thomas J, Vié B, Kluin-Nelemans JC, Viseu F, Baars JW, Poortmans P, Lugtenburg PJ, Carrie C, Jaubert J, Henry-Amar M, EORTC-GELA H8 Trial (2007) Chemotherapy plus involved-field radiation in early-stage Hodgkin’s disease. N Engl J Med 357(19):1916–27. https://doi.org/10.1056/NEJMoa064601
Engert A, Schiller P, Josting A, Herrmann R, Koch P, Sieber M, Boissevain F, De Wit M, Mezger J, Duhmke E, Willich N, Muller RP, Schmidt BF, Renner H, Muller-Hermelink HK, Pfistner B, Wolf J, Hasenclever D, Loffler M, Diehl V, German Hodgkin’s Lymphoma Study Group (2003) Involved-field radiotherapy is equally effective and less toxic compared with extended-field radiotherapy after four cycles of chemotherapy in patients with early-stage unfavorable Hodgkin’s lymphoma: results of the HD8 trial of the German Hodgkin’s Lymphoma Study Group. J Clin Oncol 21(19):3601–8. https://doi.org/10.1200/JCO.2003.03.023
Borchmann P, Eichenauer DA, Pluetschow A (2015) Targeted BEACOPP variants in patients with newly diagnosed advanced stage classical Hodgkin lymphoma: final analysis of a randomized phase II study. Blood 126:580. https://doi.org/10.1182/blood.V126.23.580.580
Hasenclever D, Loeffler M, Diehl V (1996) Rationale for dose escalation of first line conventional chemotherapy in advanced Hodgkin’s disease. German Hodgkin’s Lymphoma Study Group. Ann Oncol 7(Suppl 4):95–98. https://doi.org/10.1093/annonc/7.suppl_4.S95
Russo F, Corazzelli G, Frigeri F, Capobianco G, Aloj L, Volzone F, De Chiara A, Bonelli A, Gatani T, Marcacci G, Donnarumma D, Becchimanzi C, de Lutio E, Ionna F, De Filippi R, Lastoria S, Pinto A (2014) A phase II study of dose-dense and dose-intense ABVD (ABVDDD-DI ) without consolidation radiotherapy in patients with advanced Hodgkin lymphoma. Br J Haematol 166(1):118–129. https://doi.org/10.1111/bjh.12862
Eich HT, Diehl V, Görgen H, Pabst T, Markova J, Debus J, Ho A, Dörken B, Rank A, Grosu AL, Wiegel T, Karstens JH, Greil R, Willich N, Schmidberger H, Döhner H, Borchmann P, Müller-Hermelink HK, Müller RP, Engert A (2010) Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD11 trial. J Clin Oncol 28(27):4199–4206. https://doi.org/10.1200/JCO.2010.29.8018
Fermé C, Thomas J, Brice P, Casasnovas O, Vranovsky A, Bologna S, Lugtenburg PJ, Bouabdallah R, Carde P, Sebban C, Eghbali H, Salles G, van Imhoff GW, Thyss A, Noordijk EM, Reman O, Lybeert MLM, Janvier M, Spina M, Audhuy B, Raemaekers JMM, Delarue R, Anglaret B, de Weerdt O, Marjanovic Z, Tersteeg RJHA, de Jong D, Brière J, Henry-Amar M, European Organisation for Research and Treatment of Cancer Lymphoma Group, and; Grouped’Étude des Lymphomes de l’Adulte (2017) ABVD or BEACOPPbaseline along with involved-field radiotherapy in early-stage Hodgkin Lymphoma with risk factors: results of the European Organisation for Research and Treatment of Cancer (EORTC)-Groupe d’Étude des Lymphomes de l’Adulte (GELA) H9-U intergroup randomised trial. Eur J Cancer 81:45–55. https://doi.org/10.1016/j.ejca.2017.05.005
von Tresckow B, Plütschow A, Fuchs M, Klimm B, Markova J, Lohri A, Kral Z, Greil R, Topp MS, Meissner J, Zijlstra JM, Soekler M, Stein H, Eich HT, Mueller RP, Diehl V, Borchmann P, Engert A (2012) Dose-intensification in early unfavorable Hodgkin’s lymphoma: final analysis of the German Hodgkin Study Group HD14 trial. J Clin Oncol 30(9):907–913. https://doi.org/10.1200/JCO.2011.38.5807
André MPE, Girinsky T, Federico M (2017) Early positron emission tomography response-adapted treatment in stage I and II Hodgkin lymphoma: final results of the randomized EORTC/LYSA/FIL H10 trial. J Clin Oncol 35:1786–1794. https://doi.org/10.1200/JCO.2016.68.6394
Cottereau AS, Versari A, Loft A, Casasnovas O, Bellei M, Ricci R, Bardet S, Castagnoli A, Brice P, Raemaekers J, Deau B, Fortpied C, Raveloarivahy T, Van Zele E, Chartier L, Vander Borght T, Federico M, Hutchings M, Ricardi U, Andre M, Meignan M (2018) Prognostic value of baseline metabolic tumor volume in early-stage Hodgkin lymphoma in the standard arm of the H10 trial. Blood 131(13):1456–1463. https://doi.org/10.1182/blood-2017-07-795476
André ISHL11. 2018 Available at: https://www.hodgkinsymposium.org/videos (accessed 13 May 2020).
Cerci JJ, Pracchia LF, Linardi CC, Pitella FA, Delbeke D, Izaki M, Trindade E, Soares J Jr, Buccheri V, Meneghetti JC (2010) 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med 51(9):1337–1343. https://doi.org/10.2967/jnumed.109.073197
Jaffe ES (2009) The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program 2009:523–531. https://doi.org/10.1182/asheducation.V2009.1.523.0010523
Klimm B, Goergen H, Fuchs M, von Tresckow B, Böll B, Meissner J, Glunz A, Diehl V, Eich HT, Engert A, Borchmann P (2013) Impact of risk factors on outcomes in early-stage Hodgkin’s lymphoma: an analysis of international staging definitions. Ann Oncol 24(12):3070–3076. https://doi.org/10.1093/annonc/mdt413
Girinsky T, Specht L, Ghalibafian M, Edeline V, Bonniaud G, Van Der Maazen R, Aleman B, Paumier A, Meijnders P, Lievens Y, Noordijk E, Poortmans P, EORTC-GELA Lymphoma Group (2008) The conundrum of Hodgkin lymphoma nodes: to be or not to be included in the involved node radiation fields. The EORTC-GELA lymphoma group guidelines. Radiother Oncol 88(2):202–10. https://doi.org/10.1016/j.radonc.2008.05.012
Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español de MédulaÓsea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 32(27):3059–68. https://doi.org/10.1200/JCO.2013.54.8800
CTCAE version 4.0. Available at http://ctep.cancer.gov/reporting/ctc.html (Accessed 13 May 2020).
Hryniuk W. The importance of dose intensity in outcome of chemotherapy. In: Hellman S, De vita V, Rosenbergs SA (Eds): Important Advance in Oncology. Lippincott, Philadelphia, PA, USA, 1988, 121–124.
Simontacchi G, Filippi AR, Ciammella P, Buglione M, Saieva C, Magrini SM, Livi L, Iotti C, Botto B, Vaggelli L, Re A, Merli F, Ricardi U (2015) Interim PET after two ABVD cycles in early-stage Hodgkin lymphoma: outcomes following the continuation of chemotherapy plus radiotherapy. Int J Radiat Oncol Biol Phys 92(5):1077–1083. https://doi.org/10.1016/j.ijrobp.2015.04.021
Fleiss JL. Statistical Methods for Rates and Proportions, 1981, pp. 13–15.
Fornecker LM, Lazarovici J, Aurer I, Casasnovas RO, Gac AC, Bonnet C, Bouabdallah K, Perrot A, Specht L, Touati M, Eisenmann JC, Borel C, Stamatoullas A, Nicolas-Virelizier E, Pascal L, Lugtenburg P, Bellei M, Traverse-Glehen A, Copie C, Hutchings M, Versari A, Meignan M, Federico M, Andre M (2017) PET-based response after 2 cycles of brentuximab vedotin in combination with AVD for first-line treatment of unfavorable early-stage Hodgkin lymphoma: first analysis of the primary endpoint of breach, a randomized phase II trial of Lysa-FIL-EORTC Intergroup. Blood 130(Supplement 1):736 abstract 624.
Abramson JS, Arnason JE, LaCasce AS, Redd R, Barnes JA, Sokol L, Joyce R, Avigan D, Neuberg D, Takvorian RW, Hochberg EP, Bello CM (2019) Brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine for nonbulky limited-stage classical Hodgkin lymphoma. Blood 134(7):606–613. https://doi.org/10.1182/blood.2019001272
Gallamini A, Rigacci L, Merli F, Nassi L, Bosi A, Capodanno I, Luminari S, Vitolo U, Sancetta R, Iannitto E, Trentin L, Stelitano C, Tavera S, Biggi A, Castagnoli A, Versari A, Gregianin M, Pelosi E, Torchio P, Levis A (2006) The predictive value of positron emission tomography scanning performed after two courses of standard therapy on treatment outcome in advanced stage Hodgkin’s disease. Haematologica 91(4):475–481
Gallamini A, Hutchings M, Rigacci L, Specht L, Merli F, Hansen M, Patti C, Loft A, Di Raimondo F, D’Amore F, Biggi A, Vitolo U, Stelitano C, Sancetta R, Trentin L, Luminari S, Iannitto E, Viviani S, Pierri I, Levis A (2007) Early interim 2-[18F]fluoro-2-deoxy-D-glucose positron emission tomography is prognostically superior to international prognostic score in advanced-stage Hodgkin’s lymphoma: a report from a joint Italian-Danish study. J Clin Oncol 25(24):3746–3752. https://doi.org/10.1200/JCO.2007.11.6525
Hutchings M, Loft A, Hansen M, Pedersen LM, Buhl T, Jurlander J, Buus S, Keiding S, D’Amore F, Boesen AM, Berthelsen AK, Specht L (2006) FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood 107(1):52–59. https://doi.org/10.1182/blood-2005-06-2252
Radford J, Illidge T, Counsell N, Hancock B, Pettengell R, Johnson P, Wimperis J, Culligan D, Popova B, Smith P, McMillan A, Brownell A, Kruger A, Lister A, Hoskin P, O’Doherty M, Barrington S (2015) Results of a trial of PET-directed therapy for early-stage Hodgkin’s lymphoma. N Engl J Med 372(17):1598–1607. https://doi.org/10.1056/NEJMoa1408648
Straus DJ, Jung SH, Pitcher B, Kostakoglu L, Grecula JC, Hsi ED, Schöder H, Popplewell LL, Chang JE, Moskowitz CH, Wagner-Johnston N, Leonard JP, Friedberg JW, Kahl BS, Cheson BD, Bartlett NL (2018) CALGB 50604: risk-adapted treatment of nonbulky early-stage Hodgkin lymphoma based on interim PET. Blood 132(10):1013–1021. https://doi.org/10.1182/blood-2018-01-827246
Skoetz N, Will A, Monsef I, Brillant C, Engert A, von Tresckow B (2017) Comparison of first-line chemotherapy including escalated BEACOPP versus chemotherapy including ABVD for people with early unfavourable or advanced stage Hodgkin lymphoma. Cochrane Database Syst Rev 5(5):CD007941. doi: https://doi.org/10.1002/14651858.CD007941.pub3.
Acknowledgements
Many thanks go to Samantha Pozzi, Gianluca Gaidano, Barbara Botto, Francesco Passamonti, Emanuele Angelucci, Roberto Freilone, Pier Paolo Fattori, Lucilla Tedeschi, Francesca Re, Annalia Molinari, and Carmine Selleri, who participated to the study. The reviewers of the PET/CT scans were Antonio Castagnoli, Eugenio Borsatti, Manjola Dona, Federica Elisei, Umberto Ficola, Marcello Rodari, and Mauro Sacchetti. The authors thank Luca Giacomelli, PhD, Ambra Corti, and Aashni Shah (Polistudium) for editorial assistance, funded by internal funds.
Author information
Authors and Affiliations
Contributions
Conceptualization: AS, RM, and FM. Methodology: AS and RM. Formal analysis: LG. Investigation: All. Data curation: LG. Writing—original draft preparation: AS and RM. Writing—review and editing: All.
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the ethical committees of the participating centers.
Consent to participate
All patients provided informed consent for the inclusion in the study.
Consent for publication
All authors agreed to submit the current version of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Santoro, A., Mazza, R., Spina, M. et al. Dose-dense ABVD as first-line therapy in early-stage unfavorable Hodgkin lymphoma: results of a prospective, multicenter double-step phase II study by Fondazione Italiana Linfomi. Ann Hematol 100, 2547–2556 (2021). https://doi.org/10.1007/s00277-021-04604-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04604-x