Abstract
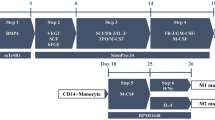
Extracellular vesicles (EVs) are bioactive, submicron-sized membrane vesicles released from all cell types upon activation or apoptosis. EVs including microparticles (MPs) and exosomes have emerged as important mediators of cell-to-cell communication in both normal and pathological states including thalassemia (thal). However, the role of EVs derived from β-thal patients with iron overload (+ IO) and without iron overload (-IO) on cardiac cells is unclear. We hypothesized plasma EVs in thal patients containing ferritin (iron storage protein) and a denaturated hemoglobin-hemichrome that induce cardiac cell proliferation. The origins and numbers of EVs isolated from plasma of normal, thal (+ IO), and (− IO) patients were compared and determined for their iron and iron-containing proteins along with their effects on cardiac and endothelial cells. Data shows that MPs were originated from many cell sources with marked numbers of platelet origin. Only the number of RBC-derived MPs in thal (+ IO) patients was significantly high when compared to normal controls. Although MPs derived from both normal and thal patients promoted cardiac cell proliferation in a dose-dependent manner, only exosomes from thal patients promoted cardiac cell proliferation compared to the untreated. Moreover, the exosomes from thal (+ IO) potentially induce higher cardiac cell proliferation and angiogenesis in terms of tube number than thal (− IO) and normal controls. Interestingly, ferritin content in the exosomes isolated from thal (+ IO) was higher than that found in the MPs isolated from the same patient. The exosomes of thal patients with higher serum ferritin level also contained greater level of ferritin inside the exosomes. Apart from ferritin, there were trends of increasing hemichrome and iron presented in the plasma EVs and EV-treated H9C2 cells. Findings from this study support the hypothesis that EVs from β-thal patients carry iron-load proteins that leads to the induction of cardiac cell proliferation.







Similar content being viewed by others
References
Weatherall DJ (1998) Thalassemia in the next millennium. Keynote address. Ann NY Acad Sci 850:1–9
Wasi P, Pootrakul S, Pootrakul P, Pravatmuang P, Winichagoon P, Fucharoen S (1980) Thalassemia in Thailand. Ann NY Acad Sci 344(1):352–363
Rund D, Rachmilewitz E (2005) β-Thalassemia. N Engl J Med 353(11):1135–1146
Taher AT, Saliba AN (2017) Iron overload in thalassemia: different organs at different rates. Hematol-Am Soc Hemat 1:265–271
Olivieri NF, Nathan DG, MacMillan JH, Wayne AS, Liu PP, McGee A et al (1994) Survival in medically treated patients with homozygous beta-thalassemia. N Engl J Med 331(9):574–578
Jaiswal R, Sedger LM (2019) Intercellular vesicular transfer by exosomes, microparticles and oncosomes - implications for cancer biology and treatments. Front Oncol 9:125
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19
Italiano JE Jr, Mairuhu AT, Flaumenhaft R (2010) Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol 17(6):578–584
Rubin O, Canellini G, Delobel J, Lion N, Tissot JD (2012) Red blood cell microparticles: clinical relevance. Transfus Med Hemother 39(5):342–347
Waldenstrom A, Genneback N, Hellman U, Ronquist G (2012) Cardiomyocyte microvesicles contain DNA/RNA and convey biological messages to target cells. PLoS One 7(4):e34653
Record M, Carayon K, Poirot M, Silvente-Poirot S (2014) Exosomes as new vesicular lipid transporters involved in cell-cell communication and various pathophysiologies. Biochim Biophys Acta. 1841(1):108–120
Tushuizen ME, Diamant M, Sturk A, Nieuwland R (2011) Cell-derived microparticles in the pathogenesis of cardiovascular disease: friend or foe? Arterioscler Thromb Vasc Biol 31(1):4–9
Konig L, Kasimir-Bauer S, Bittner AK, Hoffmann O, Wagner B, Santos Manvailer LF et al (2017) Elevated levels of extracellular vesicles are associated with therapy failure and disease progression in breast cancer patients undergoing neoadjuvant chemotherapy. Oncoimmunology 7(1):e1376153
Klaihmon P, Phongpao K, Kheansaard W, Noulsri E, Khuhapinant A, Fucharoen S et al (2017) Microparticles from splenectomized β-thalassemia/HbE patients play roles on procoagulant activities with thrombotic potential. Ann Hematol 96(2):189–198
Pattanapanyasat K, Gonwong S, Chaichompoo P, Noulsri E, Lerdwana S, Sukapirom K et al (2007) Activated platelet-derived microparticles in thalassaemia. Br J Haematol 136(3):462–471
Chaichompoo P, Kumya P, Khowawisetsut L, Chiangjong W, Chaiyarit S, Pongsakul N et al (2012) Characterizations and proteome analysis of platelet-free plasma-derived microparticles in β-thalassemia/hemoglobin E patients. J Proteomics 76:239–250
Kheansaard W, Phongpao K, Paiboonsukwong K, Pattanapanyasat K, Chaichompoo P, Svasti S (2018) Microparticles from beta-thalassaemia/HbE patients induce endothelial cell dysfunction. Sci Rep 8(1):13033
Saito H (2014) Metabolism of iron stores. Nagoya J Med Sci 76(3–4):235–254
Daru J, Colman K, Stanworth SJ, De La Salle B, Wood EM, Pasricha SR (2017) Serum ferritin as an indicator of iron status: what do we need to know? Am J Clin Nutr 106(S6):1634S-1639S
Recalcati S, Invernizzi P, Arosio P, Cairo G (2008) New functions for an iron storage protein: the role of ferritin in immunity and autoimmunity. J Autoimmun 30(1–2):84–89
Coffman LG, Parsonage D, D’Agostino R Jr, Torti FM, Torti SV (2009) Regulatory effects of ferritin on angiogenesis. PNAS 106(2):570–575
De Domenico I, Ward DM, Kaplan J (2009) Serum ferritin regulates blood vessel formation: A role beyond iron storage. PNAS 106(6):1683–1684
Pourmoghaddas A, Sanei H, Garakyaraghi M, Esteki-Ghashghaei F, Gharaati M (2014) The relation between body iron store and ferritin, and coronary artery disease. ARYA Atheroscler 10(1):32–36
Yeap BB, Divitini ML, Gunton JE, Olynyk JK, Beilby JP, McQuillan B et al (2015) Higher ferritin levels, but not serum iron or transferrin saturation, are associated with Type 2 diabetes mellitus in adult men and women free of genetic haemochromatosis. Clin Endocrinol 82(4):525–532
Salhan P, Mahajan DS, Khurana A, Kukreja S (2014) Evaluation of serum ferritin in patients of coronary artery disease. J Evol Med Dental Sci 3(72):15221–15225
Reyes C, Pons NA, Reñones CR, Gallisà JB, Val VA, Tebé C et al (2020) Association between serum ferritin and acute coronary heart disease: a population-based cohort study. Atherosclerosis 293:69–74
Pootrakul P, Vongsmasa V, La-ongpanich P, Wasi P (1981) Serum ferritin levels in thalassemias and the effect of splenectomy. Acta Haematol 66(4):244–250
Thiengtavor C, Siriworadetkun S, Paiboonsukwong K, Fucharoen S, Pattanapanyasat K, Vadolas J et al (2020) Increased ferritin levels in non-transfusion-dependent β°-thalassaemia/HbE are associated with reduced CXCR2 expression and neutrophil migration. Br J Haematol 189(1):187–198
Ferru E, Pantaleo A, Carta F, Mannu F, Khadjavi A, Gallo V et al (2014) Thalassemic erythrocytes release microparticles loaded with hemichromes by redox activation of p72Syk kinase. Haematologica 99(3):570–578
Siwaponanan P, Keawvichit R, Lekmanee K, Chomanee N, Pattanapanyasat K (2021) Enumeration and phenotyping of circulating microvesicles by flow cytometry and nanoparticle tracking analysis: plasma versus serum. Int J Lab Hematol 43(3):506–514
Winterbourn CC (1990) Oxidative reactions of hemoglobin. Methods Enzymol 186:265–272
Thery C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R et al (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 7(1):1535750
Truman-Rosentsvit M, Berenbaum D, Spektor L, Cohen LA, Belizowsy-Moshe S, Lifshitz L et al (2018) Ferritin is secreted via 2 distinct nonclassical vesicular pathways. Blood 131(3):342–352
Pattanapanyasat K, Noulsri E, Fucharoen S, Lerdwana S, Lamchiagdhase P, Siritanaratkul N et al (2004) Flow cytometric quantitation of red blood cell vesicles in thalassemia. Cytometry B Clin Cytom 57(1):23–31
Camus SM, De Moraes JA, Bonnin P, Abbyad P, Le Jeune S, Lionnet F et al (2015) Circulating cell membrane microparticles transfer heme to endothelial cells and trigger vasoocclusions in sickle cell disease. Blood 125(24):3805–3814
Kittivorapart J, Crew VK, Wilson MC, Heesom KJ, Siritanaratkul N, Toye AM (2018) Quantitative proteomics of plasma vesicles identify novel biomarkers for hemoglobin E/b-thalassemic patients. Blood Adv 2(2):95–104
Olatunya OS, Lanaro C, Longhini AL, Penteado CFF, Fertrin KY, Adekile A et al (2019) Red blood cells microparticles are associated with hemolysis markers and may contribute to clinical events among sickle cell disease patients. Ann Hematol 98(11):2507–2521
Hershko C, Peto TE (1987) Non-transferrin plasma iron. Br J Haematol 66(2):149–151
Lakhal-Littleton S (2019) Mechanisms of cardiac iron homeostasis and their importance to heart function. Free Radical Bio Med 133:234–237
Tassiopoulos T, Stamatelos G, Zakopoulos N, Fessas P, Eliopoulos GD (1995) Low incidence of acute myocardial infarction in beta-thalassaemia trait carriers. Haematologia (Budap) 26(4):199–203
Tassiopoulos S, Deftereos S, Konstantopoulos K, Farmakis D, Tsironi M, Kyriakidis M et al (2005) Does heterozygous beta-thalassemia confer a protection against coronary artery disease? Ann N Y Acad Sci 1054:467–470
Hashemi M, Shirzadi E, Talaei Z, Moghadas L, Shaygannia I, Yavari M et al (2007) Effect of heterozygous beta-thalassaemia trait on coronary atherosclerosis via coronary artery disease risk factors: a preliminary study. Cardiovasc J Afr 18(3):165–168
Tanaka Y, Schroit AJ (1983) Insertion of fluorescent phosphatidylserine into the plasma membrane of red blood cells. Recognition by autologous macrophages. J Biol Chem 258(18):11335–11343
Schroit AJ, Madsen JW, Tanaka Y (1985) In vivo recognition and clearance of red blood cells containing phosphatidylserine in their plasma membranes. J Biol Chem 260(8):5131–5138
Brancaleoni V, Di Pierro E, Motta I, Cappellini MD (2016) Laboratory diagnosis of thalassemia. Int J Lab Hematol 38(S1):32–40
Pretorius E, Vermeulen N, Bester J, du Plooy JL, Gericke GS (2014) The effect of iron overload on red blood cell morphology. Lancet 383(9918):722
Freyssinet JM (2003) Cellular microparticles: what are they bad or good for? J Thromb Haemost 1(7):1655–1662
Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G (1991) Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69(6):1476–1486
Sipido KR, Marban E (1991) L-type calcium channels, potassium channels, and novel nonspecific cation channels in a clonal muscle cell line derived from embryonic rat ventricle. Circ Res 69(6):1487–1499
Watkins SJ, Borthwick GM, Arthur HM (2011) The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. Vitro Cell Dev Biol Anim 47(2):125–131
Todorova D, Simoncini S, Lacroix R, Sabatier F, Dignat-George F (2017) Extracellular vesicles in angiogenesis. Circ Res 120(10):1658–1673
Carmeliet P, Jain RK (2011) Molecular mechanisms and clinical applications of angiogenesis. Nature 473(7347):298–307
Witek P, Korga A, Burdan F, Ostrowska M, Nosowska B, Iwan M et al (2016) The effect of a number of H9C2 rat cardiomyocytes passage on repeatability of cytotoxicity study results. Cytotechnology 68(6):2407–2415
Ménard C, Pupier S, Mornet D, Kitzmann M, Nargeot J, Lory P (1999) Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardic cells. J Biol Chem 274(41):29063–29070
Di Noto G, Chiarini M, Paolini L, Mazzoldi EL, Giustini V, Radeghieri A et al (2014) Immunoglobulin free light chains and GAGs mediate multiple myeloma extracellular vesicles uptake and secondary NfkB nuclear translocation. Front Immunol 5:517
Gonzales PA, Pisitkun T, Hoffert JD, Tchapyjnikov D, Star RA, Kleta R et al (2009) Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 20(2):363–379
Atama H, Ginsburg H (1995) Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membrane of hemoglobinopathic red blood cells. J Biol Chem 270(42):24876–24883
Serrano-Pertierra E, Oliveira-Rodriguez M, Rivas M, Oliva P, Villafani J, Navarro A et al (2019) Characterization of plasma-derived extracellular vesicles isolated by different methods: a comparison study. Bioengineering 6(1):8
Klymiuk MC, Balz N, Elashry MI, Heimann M, Wenisch S, Arnhold S (2019) Exosomes isolation and identification from equine mesenchymal stem cells. BMC Vet Res 15(1):42
Oudit GY, Sun H, Trivieri MG, Koch SE, Dawood F, Ackerley C et al (2003) L-type Ca2+ channels provide a major pathway for iron entry into cardiomyocytes in iron-overload cardiomyopathy. Nat Med 9(9):1187–1194
Mulcahy LA, Pink RC, Carter DR (2014) Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles 3:24641
Acknowledgements
The authors gratefully acknowledge the kind co-operation of thalassemia patients. We thank the primary care physicians and nurses at the Division of Hematology, Siriraj Hospital, for providing us important clinical supports. We would also like to thank Professor Aftab A. Ansari of the Department of Pathology and Laboratory, Emory University School of Medicine, Atlanta, GA, for his valuable comments and suggestions.
Funding
This work was supported by the Thailand Research Fund (TRF) – Distinguished Research Professor Grant, grant number DPG5980001, and the research grant from Faculty of Medicine Siriraj Hospital, Mahidol University. L.K was also supported by Siriraj Chalermprakiat Foundation, Faculty of Medicine Siriraj Hospital, Mahidol University.
Author information
Authors and Affiliations
Contributions
KP: research idea formation and supervision; AA: performed the experiment and data analysis; AK: patient recruitment and diagnosis; AA, PS, SS, KS, and LK: data analysis; AA and KP: manuscript writing. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the 1964 Helsinki declaration and its later amendments or comparable standards. The study was approved by Siriraj Hospital Institutional Review Board (SI-IRB), approval number Si 239/2018. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Atipimonpat, A., Siwaponanan, P., Khuhapinant, A. et al. Extracellular vesicles from thalassemia patients carry iron-containing ferritin and hemichrome that promote cardiac cell proliferation. Ann Hematol 100, 1929–1946 (2021). https://doi.org/10.1007/s00277-021-04567-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04567-z