Abstract
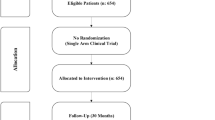
Hydroxyurea (HU) and thalidomide have been reported to improve clinical and hematological parameters in transfusion-dependent beta thalassemia (TDT). Therefore, we retrospectively analyzed the combination of HU and thalidomide in 140 transplant ineligible TDT, ≥ 10 years old, visiting our thalassemia clinic between October 2014 and November 2019. Responses were defined as maintenance of hemoglobin ≥9gm/dl without transfusion as complete response (CR) and with at least 50% reduction in transfusion burden as partial response (PR). Patients with less than 50% transfusion burden reduction for consecutive 6 months of therapy were defined as non-responders (NR), and treatment was discontinued thereafter. Primary end point was overall response rate (ORR) at last follow-up. At median follow-up of 22.6 (95% CI 16.4–28.7) months, 76 (57.2%) patients achieved CR and 19 (14.3%) achieved PR, accounting to an ORR of 71.5%. Among responders at last follow-up, a significant increase in the post-treatment hemoglobin (0.88±0.37gm/dl, p<0.0001) and drop in serum ferritin (−1490.5ng/ml, p<0.0001) were observed. Median time to CR was 124 (95% CI 75.3–172.6) days. Median longest continuous CR was 791 (95% CI 662.2–919.7) days. Common toxicities observed were sedation (25%), hyperbilirubinemia {(23.57%, grade 3/4 =17 (12.14%)}, and constipation (22.8%). Nearly three-fourth of the patients has responded with majority having CR. Adverse events are a concern; hence, regular close monitoring is a prerequisite.



Similar content being viewed by others
Data availability
Not applicable
References
Modell B, Darlison M (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86(6):480–487. https://doi.org/10.2471/blt.06.036673
Dhanya R, Sedai A, Ankita K, Parman L, Agarwal RK, Hegde S et al (2020) Life expectancy and risk factors for early death in patients with severe thalassemia syndromes in South India. Blood Adv 4(7):1448–1457. https://doi.org/10.1182/bloodadvances.2019000760
Choudhry VP, Lal A, Pati HP, Arya LS (1997) Hematological responses to hydroxyurea therapy in multi-transfused thalassemic children. Indian J Pediatr 64(3):395–398. https://doi.org/10.1007/BF02845212
Jalali Far MA, Dehghani Fard A, Hajizamani S, Mossahebi-Mohammadi M, Yaghooti H, Saki N (2016) Thalidomide is more efficient than sodium butyrate in enhancing GATA–1 and EKLF gene expression in erythroid progenitors derived from HSCs with β–globin gene mutation. Int J Hematol Oncol Stem Cell Res 10(1):37–41
Li Y, Ren Q, Zhou Y, Li P, Lin W, Yin X (2018) Thalidomide has a significant effect in patients with thalassemia intermedia. Hematology 23(1):50–54. https://doi.org/10.1080/10245332.2017.1354427
Ramanan V, Kelkar K (2017) Role of thalidomide in treatment of beta thalassemia. J Blood Disord Med 3(1):8–10. https://doi.org/10.16966/2471-5026.119
Jiskani SA, Memon S (2018) Effect of thalidomide in patients with β thalassemia major. Hematol Transfus Int J 6(6):234–236. https://doi.org/10.15406/htij.2018.06.00191
Masera N, Tavecchia L, Capra M, Cazzaniga G, Vimercati C, Pozzi L et al (2010) Optimal response to thalidomide in a patient with thalassaemia major resistant to conventional therapy. Blood Transfus 8(1):63–65. https://doi.org/10.2450/2009.0102-09
Fozza C, Pardini S, Giannico D, Targhetta C, Di Tucci AA, Dessalvi P et al (2005) Dramatic erythroid response to low-dose thalidomide in two patients with transfusion independent thalassemia and severe post-transfusional alloimmune hemolysis. Am J Hematol 90(7):E141. https://doi.org/10.1002/ajh.24030
Cappellini MD, Viprakasit V, Taher AT, Georgiev P, Kuo KH, Coates T et al (2020) A phase 3 trial of luspatercept in patients with transfusion-dependent β-thalassemia. N Engl J Med 382(13):1219–1231. https://doi.org/10.1056/NEJMoa1910182
Palumbo A, Palladino C (2012) Venous and arterial thrombotic risks with thalidomide: evidence and practical guidance. Ther Adv Drug Saf 3(5):255–266
NIH National Cancer Institute. Common Terminology Criteria for adverse events (CTCAE) Version 5.0. November 27, 2017
Taher AT, Musallam KH, Cappellini MD (2021) β Thalassemias. N Eng J Med 384(8):727–743. https://doi.org/10.1056/NEJMra2021838
Charache S, Terrin ML, Moore RD, Dover GJ, Barton FB, Eckert SV, McMahon RP, Bonds DR (1995) Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. N Engl J Med 332(20):1317–1322. https://doi.org/10.1056/NEJM199505183322001
Aguilar-Lopez LB, Delgado-Lamas JL, Rubio-Jurado B, Perea FJ, Ibarra B (2008) Thalidomide therapy in a patient with thalassemia major. Blood Cells Mol Dis 41(1):136–137. https://doi.org/10.1016/j.bcmd.2008.03.001
Nag A, Radhakrishnan VS, Kumar J, Bhave S, Mishra DK, Nair R, Chandy M (2020) Thalidomide in patients with transfusion-dependent E-beta thalassemia refractory to hydroxyurea: a single-center experience. Indian J Hematol Blood Transfus 36(2):399–402. https://doi.org/10.1007/s12288-020-01263-2
Yassin AK (2020) Promising response to thalidomide in symptomatic b-thalassemia. Indian J Hematol Blood Transfus 36(2):337–341. https://doi.org/10.1007/s12288-019-01231-5
Chen J, Zhu W, Cai N, Bu S, Li J, Huang L (2017) Thalidomide induces haematologic responses in patients with β-thalassaemia. Eur J Haematol 99:437–441. https://doi.org/10.1111/ejh.12955
Yang K, Wu Y, Zhou Y, Long B, Lu Q, Zhou T, Wang L, Geng Z, Yin X (2020) Thalidomide for patients with β-thalassemia: a multicenter experience. Mediterr J Hematol Infect Dis 12(1):e2020021. https://doi.org/10.4084/MJHID.2020.021
Begum M, Moslem MHM, Begum NNF, Rahman MZ (2020) Outcome of treatment with thalidomide in transfusion dependent thalassemia patients: a prospective study in a Thalassemia Center, Dhaka, Bangladesh. Am J Pediatr 6(3):168–171. https://doi.org/10.11648/j.ajp.20200603.11
Shah S, Sheth R, Shah K, Patel K (2020) Safety and effectiveness of thalidomide and hydroxyurea combination in β-thalassaemia intermedia and major: a retrospective pilot study. Br J Haematol 188:e18–e21. https://doi.org/10.1111/bjh.16272
Acknowledgements
We would like to acknowledge Dr Priyanka Soni in initial data collection. We are indebted to Dr Anurag Sharma, Department of Research, Rajiv Gandhi Cancer Institute and Research Centre, Delhi, India, for providing the guidance in the statistical assessment. We also like to acknowledge all the staff of Department of Hematology and BMT unit, Rajiv Gandhi Cancer Institute and Research Centre, for providing the support in conducting the study.
Code availability
Not applicable
Author information
Authors and Affiliations
Contributions
DB conceptualized, interpreted, and contributed in writing the manuscript. JK collected, analyzed, and interpreted the data and contributed in writing the manuscript. NY collected the data. PM interpreted the data and was a major contributor in writing the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All authors stated that the study has been approved by the appropriate institutional review board and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Since this is a retrospective study, hence no formal informed consent is required.
Consent to participate
No formal consent was taken for participation in the study.
Consent for publication
Not applicable
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 34 kb)
Rights and permissions
About this article
Cite this article
Bhurani, D., Kapoor, J., Yadav, N. et al. Experience with combination of hydroxyurea and low-dose thalidomide in transfusion-dependent beta thalassemia patients. Ann Hematol 100, 1417–1427 (2021). https://doi.org/10.1007/s00277-021-04501-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-021-04501-3