Abstract
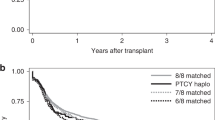
There are a limited number of studies comparing outcomes of busulfan (BU)-based myeloablative hematopoietic stem cell transplantation using unmanipulated haploidentical donors (HIDs), HLA-matched unrelated donors (MUDs), and HLA-matched sibling related donors (MSDs) in acute myeloid leukemia (AML) patients with complete remission (CR) status. With this background, we compared outcomes among 377 cases of CR following consecutive HID-HSCT for AML (CR) to 86 MUD and 92 MSD-HSCT cases. All patients received BU-based myeloablative conditioning and an unmanipulated graft within the same period. The median patient age was 23 years (range 1.1 to 65 years), and 230 patients (41.4%) were under age18. Among the 555 patients, 432 (77.8%) were of intermediate cytogenetic risk and 123 (22.2%) were of adverse risk. A total of 113 patients (20.5%) had FLT3-ITD+ AML, 425 patients (76.6%) were in first complete remission (CR1) post-transplant, and 130 (23.4%) patients were in second CR (CR2). GVHD prophylaxis included mycophenolate mofetil (MMF), cyclosporine-A (CSA) with short-term methotrexate (MTX) for HID, and MUD-HSCT. MMF is not used for MSD-HSCT. The median survival follow-up time was 42 months (range 18–91 months). The 3-year leukemia-free survival (LFS) among the HID, MUD, and MSD cohorts was 73.8% ± 4.8%, 66.4% ± 8.5%, 74.5% ± 2.4%, respectively (P = 0.637). Three-year overall survival (OS) was 74.9% ± 2.4%, 81.8% ± 4.3%, and 77.5% ± 4.5% among the HID, MUD, and MSD cohorts, respectively (P = 0.322). There were no difference among the relapse rate among the HID, MUD, and MSD donor cohorts (14.3% ± 4.0% vs 20.3% ± 6.4% vs 14.5% ± 2.2, respectively; P = 0.851) or the non-relapse mortality (NRM) (12.3% ± 3.5% vs 9.5% ± 3.2% vs 14.0% ± 1.8%, respectively; P = 0.441). Multivariate analyses showed that MRD-positive pre-HSCT was the only risk factor associated with a lower OS and LFS and higher risk of relapse among all 555 patients. Compared with the use of a MUD or MSD, an HID for HSCT had similar outcomes among AML patients with CR states who underwent an allo-HSCT with BU-based myeloablative conditioning. MFC-MRD-positive pre-HSCT was an independent negative factor impact on outcomes for AML patients in CR. We conclude that for AML patients who do not have a MSD or if an urgent transplant is required, HSCT from an HID is a valid option.



Similar content being viewed by others
References
NCCN Clinical Practice Guidelines in Oncology. Acute myeloid leukemia. Version 3. 2020. (Guidelines)
Versluis J, Labopin M, Ruggeri A, Socie G et al (2017) Alternative donors for allogeneic hematopoietic stem cell transplantation in poor-risk AML in CR1. Blood Adv 1(7):477–485
Giebel S, Boumendil A, Labopin M (2019) Trends in the use of hematopoietic stem cell transplantation for adults with acute lymphoblastic leukemia in Europe: a report from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation (EBMT). Ann Hematol 98:2389–2398
Rashidi A, Slade M, DiPersio JF, Westervelt P et al (2016) Post-transplant high-dose cyclophosphamide after HLA-matched vs haploidentical hematopoietic cell transplantation for AML. Bone Marrow Transplant 51:1561–1564
Bashey A, Xu Z, Jackson K et al (2016) Comparison of outcomes of hematopoietic cell transplants from T-replete haploidentical donors using post-transplantation cyclophosphamide with 10 of 10 HLA-A, -B, -C, -DRB1, and -DQB1 allele-matched unrelated donors and HLA-identical sibling donors: a multivariable analysis including disease risk index. Biol Blood Marrow Transplant 22:125e133
Arber DA, Orazi A, Hasserjian R et al (2016) The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 127(20):2391–2405
Yu W, Chang Y-J, Xu L-P et al (2014) Who is the best donor for a related HLA haplotype-mismatched transplant? Blood. 124(6):843–850
Slovak ML, Kopecky KJ, Peter A. Cassileth, et al. (2000) Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group study. Blood. 96(13):4075–4083
Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an International Expert Panel. Blood 129(4):424–447. https://doi.org/10.1182/blood-2016-08-733196
Sorror ML, Maris MB, Storb R et al (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 106(8):2912–2919
Stefan O Ciurea , Kai Cao, Marcelo Fernandez-Vina, Piyanuch Kongtim, et al. The European Society for Blood and Marrow Transplantation (EBMT) consensus guidelines for the detection and treatment of donor-specific anti-HLA antibodies (DSA) in haploidentical hematopoietic cell transplantation. Bone Marrow Transplant2018;53(5):521-534.
Lu D-P, Dong L, Wu T, Huang X-J et al (2006) Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 107(8):3065–3073
Harris AC, Young R, Devine S et al (2016) International, multicenter standardization of acute graft-versus-host disease clinical data collection: a report from the Mount Sinai Acute GVHD International Consortium. Biol Blood Marrow Transplant 22:4e10
Jagasia MH, Hildegard T, Greinix MA et al (2015) National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 Diagnosis and Staging Working Group Report. Biol Blood Marrow Transplant 21(3):389–401
Di Stasi A, Milton DR, Poon LM et al (2014) Similar transplant outcomes for AML/MDS patients with haploidentical versus 10/10 HLA matched unrelated and related donors. Biol Blood Marrow Transplant 20(12):1975–1981
Yu W, Liu Q-F, Xu L-P, Liu K-Y et al (2015) Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 125(25):3956–3962
Salvatore D, Labopin M, Ruggeri A et al (2018) Outcomes of hematopoietic stem cell transplantation from unmanipulated haploidentical versus matched sibling donor in patients with acute myeloid leukemia in first complete remission with intermediate or high-risk cytogenetics: a study from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica 103(8):1317–1328
Ringdén O, Labopin M, Ciceri F, Velardi A et al (2016) Is there a stronger graft-versus-leukemia effect using HLA haploidentical donors than with HLA-identical siblings? Leukemia. 30(2):447–455
Chang Y-J, Yu W, Liu Y-R, Xu L-P et al (2017) Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol 10(1):134
Yu W, Liu D-H, Xu L-P, Liu K-Y et al (2011 Jun) Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 17(6):821–830
Getta BM, Devlin SM, Levine RL et al (2017) Multicolor flow cytometry and multigene next-generation sequencing are complementary and highly predictive for relapse in acute myeloid leukemia after allogeneic transplantation. Biol Blood Marrow Transplant 23:1064–1071
Feng S, Zhou L, Zhang X, Tang B, Zhu X et al (2019) Impact of ELN risk stratification, induction chemotherapy regimens and hematopoietic stem cell transplantation on outcomes in hyperleukocytic acute myeloid leukemia with initial white blood cell count more than 100 × 109/L. Cancer Manag Res 11:9495–9503
Schmaelter A-K, Labopin M, Socié G, Itälä-Remes M et al (2020) Inferior outcome of allogeneic stem cell transplantation for secondary acute myeloid leukemia in first complete remission as compared to de novo acute myeloid leukemia. Blood Cancer J 10(3):26
Ning Xie MD, Jian Zhou PD, Yanli Zhang MD et al (2019 May) Extramedullary relapse of leukemia after allogeneic hematopoietic stem cell transplantation: a retrospective study. Medicine (Baltimore) 98(19):e15584
Okamoto Y, Kudo K, Tabuchi K, Tomizawa D et al (2019 Sep) Hematopoietic stem-cell transplantation in children with refractory acute myeloid leukemia. Bone Marrow Transplant 54(9):1489–1498
Walter RB, Buckley SA, John M. Pagel, et al. (2013) Significance of minimal residual disease before myeloablative allogeneic hematopoietic cell transplantation for AML in first and second complete remission. Blood. 122(10):1813–1821
Walter RB, Gooley TA, Wood BL, Milano F et al (2011) Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. J Clin Oncol 29(9):1190–1197
Raiola AM, Dominietto A, di Grazia C et al (2014) Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 20:1573e1579
Miflin G, Russell NH, Franklin I, Cook G, Milligan DW et al (2000) An analysis of the effect of chronic GvHD on relapse and survival following allogeneic PBSC transplantation. Cytotherapy. 2(6):423–428
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lu, Y., Zhao, YL., Zhang, JP. et al. Comparable outcomes among unmanipulated haploidentical, matched unrelated, and matched sibling donors in BU-based myeloablative hematopoietic stem cell transplantation for intermediate and adverse risk acute myeloid leukemia in complete remission: a single-center study. Ann Hematol 100, 1579–1591 (2021). https://doi.org/10.1007/s00277-020-04355-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04355-1