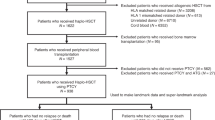
Abstract
The purpose of our study is to identify the efficacy of ruxolitinib in human leukocyte antigen (HLA) haploidentical hematopoietic stem cell transplantation (haplo-HSCT) recipients with multidrug-resistant (MDR)-graft-versus-host disease (GVHD, n = 34). MDR-GVHD was defined as GVHD showing no improvement after at least 3 types of treatments. The median number of previous GVHD-therapies was 4 for both MDR-acute GVHD (aGVHD) and MDR-chronic GVHD (cGVHD). For MDR-aGVHD (n = 15), the median time to response was 10 days (range 2 to 65), and the overall response rate (ORR) was 60.0% (9/15), including 40.0% (6/15) complete response (CR) and 20.0% (3/15) partial response (PR). The 1-year probability of overall survival after ruxolitinib was 66.7%. The rates of hematologic and infectious toxicities were 73.3% and 46.7% after ruxolitinib treatment. For MDR-cGVHD (n = 19), the median time to response was 29 days (range 6 to 175), and the ORR was 89.5% (17/19), including 26.3% (5/19) CR and 63.2% (12/19) PR. All patients remained alive until our last follow-up. The rates of hematologic and infectious toxicities were 36.8% and 47.4% after ruxolitinib treatment. Ruxolitinib is an effective salvage treatment for MDR-GVHD in haplo-HSCT recipients.



Similar content being viewed by others
References
Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, Liu D, Liu Q, Liu T, Jiang M, Ren H, Song Y, Sun Z, Wang J, Wu D, Zhou D, Zou P, Liu K, Huang X (2018) The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol 11(1):33–33. https://doi.org/10.1186/s13045-018-0564-x
Deeg HJ, Henslee-Downey PJ (1990) Management of acute graft-versus-host disease. Bone Marrow Transplant 6(1):1–8
Ferrara JL, Levine JE, Reddy P, Holler E (2009) Graft-versus-host disease. Lancet 373(9674):1550–1561. https://doi.org/10.1016/s0140-6736(09)60237-3
Garnett C, Apperley JF, Pavlu J (2013) Treatment and management of graft-versus-host disease: improving response and survival. Ther Adv Hematol 4(6):366–378. https://doi.org/10.1177/2040620713489842
Khoury H, Kashyap A, Adkins DR, Brown RA, Miller G, Vij R, Westervelt P, Trinkaus K, Goodnough LT, Hayashi RJ, Parker P, Forman SJ, DiPersio JF (2001) Treatment of steroid-resistant acute graft-versus-host disease with anti-thymocyte globulin. Bone Marrow Transplant 27(10):1059–1064. https://doi.org/10.1038/sj.bmt.1703032
Martin PJ, Rizzo JD, Wingard JR, Ballen K, Curtin PT, Cutler C, Litzow MR, Nieto Y, Savani BN, Schriber JR, Shaughnessy PJ, Wall DA, Carpenter PA (2012) First- and second-line systemic treatment of acute graft-versus-host disease: recommendations of the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant 18(8):1150–1163. https://doi.org/10.1016/j.bbmt.2012.04.005
Schmidt-Hieber M, Fietz T, Knauf W, Uharek L, Hopfenmuller W, Thiel E, Blau IW (2005) Efficacy of the interleukin-2 receptor antagonist basiliximab in steroid-refractory acute graft-versus-host disease. Br J Haematol 130(4):568–574. https://doi.org/10.1111/j.1365-2141.2005.05631.x
Liu S-N, Xu L-P, Zhang X-H, Wang Y, Yan C-H, Chen Y-H, Chen H, Han W, Wang F-R, Wang J-Z, Tang F-F, Cheng Y-F, Liu K-Y, Huang X-J, Mo X-D (2020) Prognostic factors and long-term follow-up of basiliximab for steroid-refractory acute graft-versus-host disease: updated experiences from a large-scale study. Am J Hematol Accepted
Magenau J, Runaas L, Reddy P (2016) Advances in understanding the pathogenesis of graft-versus-host disease. Br J Haematol 173(2):190–205. https://doi.org/10.1111/bjh.13959
Zeiser R, Blazar BR (2017) Acute graft-versus-host disease - biologic process, prevention, and therapy. N Engl J Med 377(22):2167–2179. https://doi.org/10.1056/NEJMra1609337
Zeiser R, Blazar BR (2017) Pathophysiology of chronic graft-versus-host disease and therapeutic targets. N Engl J Med 377(26):2565–2579. https://doi.org/10.1056/NEJMra1703472
Schroeder MA, Choi J, Staser K, DiPersio JF (2018) The Role of Janus Kinase Signaling in Graft-Versus-Host Disease and Graft Versus Leukemia. Biol Blood Marrow Transplant 24(6):1125–1134. https://doi.org/10.1016/j.bbmt.2017.12.797
Spoerl S, Mathew NR, Bscheider M, Schmitt-Graeff A, Chen S, Mueller T, Verbeek M, Fischer J, Otten V, Schmickl M, Maas-Bauer K, Finke J, Peschel C, Duyster J, Poeck H, Zeiser R, von Bubnoff N (2014) Activity of therapeutic JAK 1/2 blockade in graft-versus-host disease. Blood 123(24):3832–3842. https://doi.org/10.1182/blood-2013-12-543736
Abedin S, McKenna E, Chhabra S, Pasquini M, Shah NN, Jerkins J, Baim A, Runaas L, Longo W, Drobyski W, Hari PN, Hamadani M (2019) Efficacy, Toxicity, and infectious complications in ruxolitinib-treated patients with corticosteroid-refractory graft-versus-host disease after hematopoietic cell transplantation. Biol Blood Marrow Transplant 25(8):1689–1694. https://doi.org/10.1016/j.bbmt.2019.04.003
Ferreira AM, Pontes da Silva CA, Pereira AD, Szor RS, Medeiros da Fonseca ARB, Serpa MG, Xavier EM, Sampaio de Melo MK, Novis Y, Tucunduva L, Rocha V, Arrais-Rodrigues C (2018) Ruxolitinib in steroid-refractory chronic graft-versus-host disease: experience of a single center. Bone Marrow Transplant 53(4):503–506. https://doi.org/10.1038/s41409-017-0068-2
Khandelwal P, Teusink-Cross A, Davies SM, Nelson AS, Dandoy CE, El-Bietar J, Marsh RA, Kumar AR, Grimley MS, Jodele S, Myers KC (2017) Ruxolitinib as salvage therapy in steroid-refractory acute graft-versus-host disease in pediatric hematopoietic stem cell transplant patients. Biol Blood Marrow Transplant 23(7):1122–1127. https://doi.org/10.1016/j.bbmt.2017.03.029
Khoury HJ, Langston AA, Kota VK, Wilkinson JA, Pusic I, Jillella A, Bauer S, Kim AS, Roberts D, Al-Kadhimi Z, Bodo I, Winton E, Arellano M, DiPersio JF (2018) Ruxolitinib: a steroid sparing agent in chronic graft-versus-host disease. Bone Marrow Transplant 53(7):826–831. https://doi.org/10.1038/s41409-017-0081-5
Sarmiento Maldonado M, Ramirez Villanueva P, Bertin Cortes-Monroy P, Jara Arias V, Soto Donoso K, Uribe Gonzalez P, Ocqueteau Tachini M, Perez-Simon JA (2017) Compassionate use of ruxolitinib in acute and chronic graft versus host disease refractory both to corticosteroids and extracorporeal photopheresis. Exp Hematol Oncol 6:32. https://doi.org/10.1186/s40164-017-0092-3
Zeiser R, Burchert A, Lengerke C, Verbeek M, Maas-Bauer K, Metzelder SK, Spoerl S, Ditschkowski M, Ecsedi M, Sockel K, Ayuk F, Ajib S, de Fontbrune FS, Na IK, Penter L, Holtick U, Wolf D, Schuler E, Meyer E, Apostolova P, Bertz H, Marks R, Lubbert M, Wasch R, Scheid C, Stolzel F, Ordemann R, Bug G, Kobbe G, Negrin R, Brune M, Spyridonidis A, Schmitt-Graff A, van der Velden W, Huls G, Mielke S, Grigoleit GU, Kuball J, Flynn R, Ihorst G, Du J, Blazar BR, Arnold R, Kroger N, Passweg J, Halter J, Socie G, Beelen D, Peschel C, Neubauer A, Finke J, Duyster J, von Bubnoff N (2015) Ruxolitinib in corticosteroid-refractory graft-versus-host disease after allogeneic stem cell transplantation: a multicenter survey. Leukemia 29(10):2062–2068. https://doi.org/10.1038/leu.2015.212
Jagasia M, Perales MA, Schroeder MA, Ali H, Shah NN, Chen YB, Fazal S, Dawkins FW, Arbushites MC, Tian C, Connelly-Smith L, Howell MD, Khoury HJ (2020) Ruxolitinib for the treatment of steroid-refractory acute GVHD (REACH1): a multicenter, open-label, phase 2 trial. Blood 135:1739–1749. https://doi.org/10.1182/blood.2020004823
Wang Y, Wu DP, Liu QF, Xu LP, Liu KY, Zhang XH, Yu WJ, Xu Y, Huang F, Huang XJ (2019) Low-dose post-transplant cyclophosphamide and anti-thymocyte globulin as an effective strategy for GVHD prevention in haploidentical patients. J Hematol Oncol 12(1):88. https://doi.org/10.1186/s13045-019-0781-y
Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, Fan ZP, Wu DP, Huang XJ (2015) Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 125(25):3956–3962. https://doi.org/10.1182/blood-2015-02-627786
Wang Y, Wang HX, Lai YR, Sun ZM, Wu DP, Jiang M, Liu DH, Xu KL, Liu QF, Liu L, Wang JB, Gao F, Ou-Yang J, Gao SJ, Xu LP, Huang XJ (2016) Haploidentical transplant for myelodysplastic syndrome: registry-based comparison with identical sibling transplant. Leukemia 30(10):2055–2063. https://doi.org/10.1038/leu.2016.110
Sun YQ, He GL, Chang YJ, Xu LP, Zhang XH, Han W, Chen H, Chen YH, Wang Y, Wang FR, Wang JZ, Liu KY, Huang XJ (2015) The incidence, risk factors, and outcomes of primary poor graft function after unmanipulated haploidentical stem cell transplantation. Ann Hematol 94(10):1699–1705. https://doi.org/10.1007/s00277-015-2440-x
Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, Chen H, Liu DH, Gao ZY, Chen YH, Xu LP, Zhang YC, Ren HY, Li D, Liu KY (2006) Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood 107(8):3065–3073. https://doi.org/10.1182/blood-2005-05-2146
Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, Morris LE, Solomon SR (2013) T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol 31(10):1310–1316. https://doi.org/10.1200/jco.2012.44.3523
Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, McQuitty M, Hunter DS, Levy R, Knoops L, Cervantes F, Vannucchi AM, Barbui T, Barosi G (2012) JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 366(9):787–798. https://doi.org/10.1056/NEJMoa1110556
Saeed I, McLornan D, Harrison CN (2017) Managing side effects of JAK inhibitors for myelofibrosis in clinical practice. Expert Rev Hematol 10(7):617–625. https://doi.org/10.1080/17474086.2017.1337507
Xiao-Jun H, Lan-Ping X, Kai-Yan L, Dai-Hong L, Yu W, Huan C, Yu-Hong C, Wei H, Jing-Zhi W, Yao C, Xiao-Hui Z, Hong-Xia S, Feng-Rong W, Fei-Fei T (2009) Partially matched related donor transplantation can achieve outcomes comparable with unrelated donor transplantation for patients with hematologic malignancies. Clin Cancer Res 15(14):4777–4783. https://doi.org/10.1158/1078-0432.Ccr-09-0691
Mo X-D, Zhang Y-Y, Zhang X-H, Xu L-P, Wang Y, Yan C-H, Chen H, Chen Y-H, Chang Y-J, Liu K-Y, Huang X-J (2018) The role of collateral related donors in haploidentical hematopoietic stem cell transplantation. Sci Bull 63(20):1376–1382. https://doi.org/10.1016/j.scib.2018.08.008
Xu LP, Jin S, Wang SQ, Xia LH, Bai H, Gao SJ, Liu QF, Wang JM, Wang X, Jiang M, Zhang X, Wu DP, Huang XJ (2017) Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol 10(1):25. https://doi.org/10.1186/s13045-017-0398-y
Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, Chen YH, Wang JZ, Gao ZY, Zhang YC, Jiang Q, Shi HX, Lu DP (2006) Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant 38(4):291–297. https://doi.org/10.1038/sj.bmt.1705445
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, Thomas ED (1995) 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant 15(6):825–828
Dignan FL, Clark A, Amrolia P, Cornish J, Jackson G, Mahendra P, Scarisbrick JJ, Taylor PC, Hadzic N, Shaw BE, Potter MN (2012) Diagnosis and management of acute graft-versus-host disease. Br J Haematol 158(1):30–45. https://doi.org/10.1111/j.1365-2141.2012.09129.x
Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, Palmer J, Weisdorf D, Treister NS, Cheng GS, Kerr H, Stratton P, Duarte RF, McDonald GB, Inamoto Y, Vigorito A, Arai S, Datiles MB, Jacobsohn D, Heller T, Kitko CL, Mitchell SA, Martin PJ, Shulman H, Wu RS, Cutler CS, Vogelsang GB, Lee SJ, Pavletic SZ, Flowers ME (2015) National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 21(3):389–401.e381. https://doi.org/10.1016/j.bbmt.2014.12.001
Dignan FL, Amrolia P, Clark A, Cornish J, Jackson G, Mahendra P, Scarisbrick JJ, Taylor PC, Shaw BE, Potter MN (2012) Diagnosis and management of chronic graft-versus-host disease. Br J Haematol 158(1):46–61. https://doi.org/10.1111/j.1365-2141.2012.09128.x
Mo XD, Xu LP, Liu DH, Zhang XH, Chen H, Chen YH, Han W, Wang Y, Wang FR, Wang JZ, Liu KY, Huang XJ (2013) Nonmalignant late effects in survivors of partially matched donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 19(5):777–783. https://doi.org/10.1016/j.bbmt.2013.01.026
Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, Chen YH, Han W, Wang FR, Wang JZ, Liu KY, Huang XJ (2016) Late-onset severe pneumonia after allogeneic hematopoietic stem cell transplantation: prognostic factors and treatments. Transpl Infect Dis 18(4):492–503. https://doi.org/10.1111/tid.12553
Gronningsaeter IS, Tsykunova G, Lilleeng K, Ahmed AB, Bruserud O, Reikvam H (2017) Bronchiolitis obliterans syndrome in adults after allogeneic stem cell transplantation-pathophysiology, diagnostics and treatment. Expert Rev Clin Immunol 13(6):553–569. https://doi.org/10.1080/1744666x.2017.1279053
Hakim A, Cooke KR, Pavletic SZ, Khalid M, Williams KM, Hashmi SK (2019) Diagnosis and treatment of bronchiolitis obliterans syndrome accessible universally. Bone Marrow Transplant 54(3):383–392. https://doi.org/10.1038/s41409-018-0266-6
Okamoto M, Okano A, Akamatsu S, Ashihara E, Inaba T, Takenaka H, Katoh N, Kishimoto S, Shimazaki C (2006) Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia 20(1):172–173. https://doi.org/10.1038/sj.leu.2403996
Ratanatharathorn V, Ayash L, Reynolds C, Silver S, Reddy P, Becker M, Ferrara JL, Uberti JP (2003) Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant 9(8):505–511
von Bonin M, Oelschlagel U, Radke J, Stewart M, Ehninger G, Bornhauser M, Platzbecker U (2008) Treatment of chronic steroid-refractory graft-versus-host disease with low-dose rituximab. Transplantation 86(6):875–879. https://doi.org/10.1097/TP.0b013e318183f662
Zaja F, Bacigalupo A, Patriarca F, Stanzani M, Van Lint MT, Fili C, Scime R, Milone G, Falda M, Vener C, Laszlo D, Alessandrino PE, Narni F, Sica S, Olivieri A, Sperotto A, Bosi A, Bonifazi F, Fanin R (2007) Treatment of refractory chronic GVHD with rituximab: a GITMO study. Bone Marrow Transplant 40(3):273–277. https://doi.org/10.1038/sj.bmt.1705725
Olivieri A, Cimminiello M, Corradini P, Mordini N, Fedele R, Selleri C, Onida F, Patriarca F, Pavone E, Svegliati S, Gabrielli A, Bresciani P, Nuccorini R, Pascale S, Coluzzi S, Pane F, Poloni A, Olivieri J, Leoni P, Bacigalupo A (2013) Long-term outcome and prospective validation of NIH response criteria in 39 patients receiving imatinib for steroid-refractory chronic GVHD. Blood 122(25):4111–4118. https://doi.org/10.1182/blood-2013-05-494278
Schwartz DM, Bonelli M, Gadina M, O'Shea JJ (2016) Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 12(1):25–36. https://doi.org/10.1038/nrrheum.2015.167
Hechinger AK, Smith BA, Flynn R, Hanke K, McDonald-Hyman C, Taylor PA, Pfeifer D, Hackanson B, Leonhardt F, Prinz G, Dierbach H, Schmitt-Graeff A, Kovarik J, Blazar BR, Zeiser R (2015) Therapeutic activity of multiple common γ-chain cytokine inhibition in acute and chronic GVHD. Blood 125(3):570–580. https://doi.org/10.1182/blood-2014-06-581793
Yarilina A, Xu K, Chan C, Ivashkiv LB (2012) Regulation of inflammatory responses in tumor necrosis factor-activated and rheumatoid arthritis synovial macrophages by JAK inhibitors. Arthritis Rheum 64(12):3856–3866. https://doi.org/10.1002/art.37691
Funke VA, de Medeiros CR, Setubal DC, Ruiz J, Bitencourt MA, Bonfim CM, Neto JZ, Pasquini R (2006) Therapy for severe refractory acute graft-versus-host disease with basiliximab, a selective interleukin-2 receptor antagonist. Bone Marrow Transplant 37(10):961–965. https://doi.org/10.1038/sj.bmt.1705306
Wang JZ, Liu KY, Xu LP, Liu DH, Han W, Chen H, Chen YH, Zhang XH, Zhao T, Wang Y, Huang XJ (2011) Basiliximab for the treatment of steroid-refractory acute graft-versus-host disease after unmanipulated HLA-mismatched/haploidentical hematopoietic stem cell transplantation. Transplant Proc 43(5):1928–1933. https://doi.org/10.1016/j.transproceed.2011.03.044
Snover DC, Weisdorf SA, Ramsay NK, McGlave P, Kersey JH (1984) Hepatic graft versus host disease: a study of the predictive value of liver biopsy in diagnosis. Hepatology 4(1):123–130. https://doi.org/10.1002/hep.1840040122
Shulman HM, Sharma P, Amos D, Fenster LF, McDonald GB (1988) A coded histologic study of hepatic graft-versus-host disease after human bone marrow transplantation. Hepatology 8(3):463–470. https://doi.org/10.1002/hep.1840080305
Yeh KH, Hsieh HC, Tang JL, Lin MT, Yang CH, Chen YC (1994) Severe isolated acute hepatic graft-versus-host disease with vanishing bile duct syndrome. Bone Marrow Transplant 14(2):319–321
Aliani S, Meyer S, Krenn T, Müller-Molaian I, Graf N (2002) Vanishing bile duct syndrome following allogeneic bone marrow transplantation. Klin Padiatr 214(6):371–374. https://doi.org/10.1055/s-2002-35372
Gomez-Almaguer D, Ruiz-Arguelles GJ, del Carmen T-AL, Gonzalez-Llano O, Gutierrez-Aguirre H, Cantu-Rodriguez O, Jaime-Perez J, Carrasco-Yalan A, Giralt S (2008) Alemtuzumab for the treatment of steroid-refractory acute graft-versus-host disease. Biol Blood Marrow Transplant 14(1):10–15. https://doi.org/10.1016/j.bbmt.2007.08.052
Kim JG, Sohn SK, Kim DH, Lee NY, Suh JS, Lee KS, Lee KB (2004) Different efficacy of mycophenolate mofetil as salvage treatment for acute and chronic GVHD after allogeneic stem cell transplant. Eur J Haematol 73(1):56–61. https://doi.org/10.1111/j.1600-0609.2004.00247.x
Krejci M, Doubek M, Buchler T, Brychtova Y, Vorlicek J, Mayer J (2005) Mycophenolate mofetil for the treatment of acute and chronic steroid-refractory graft-versus-host disease. Ann Hematol 84(10):681–685. https://doi.org/10.1007/s00277-005-1070-0
Martinez C, Solano C, Ferra C, Sampol A, Valcarcel D, Perez-Simon JA (2009) Alemtuzumab as treatment of steroid-refractory acute graft-versus-host disease: results of a phase II study. Biol Blood Marrow Transplant 15(5):639–642. https://doi.org/10.1016/j.bbmt.2009.01.014
Schub N, Gunther A, Schrauder A, Claviez A, Ehlert C, Gramatzki M, Repp R (2011) Therapy of steroid-refractory acute GVHD with CD52 antibody alemtuzumab is effective. Bone Marrow Transplant 46(1):143–147. https://doi.org/10.1038/bmt.2010.68
Couriel D, Saliba R, Hicks K, Ippoliti C, de Lima M, Hosing C, Khouri I, Andersson B, Gajewski J, Donato M, Anderlini P, Kontoyiannis DP, Cohen A, Martin T, Giralt S, Champlin R (2004) Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood 104(3):649–654. https://doi.org/10.1182/blood-2003-12-4241
Kharfan-Dabaja MA, Mhaskar AR, Djulbegovic B, Cutler C, Mohty M, Kumar A (2009) Efficacy of rituximab in the setting of steroid-refractory chronic graft-versus-host disease: a systematic review and meta-analysis. Biol Blood Marrow Transplant 15(9):1005–1013. https://doi.org/10.1016/j.bbmt.2009.04.003
Marty FM, Ljungman P, Chemaly RF, Maertens J, Dadwal SS, Duarte RF, Haider S, Ullmann AJ, Katayama Y, Brown J, Mullane KM, Boeckh M, Blumberg EA, Einsele H, Snydman DR, Kanda Y, DiNubile MJ, Teal VL, Wan H, Murata Y, Kartsonis NA, Leavitt RY, Badshah C (2017) Letermovir prophylaxis for cytomegalovirus in hematopoietic-cell transplantation. N Engl J Med 377(25):2433–2444. https://doi.org/10.1056/NEJMoa1706640
Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, Armand P, Antin JH, Chen J, Devine SM, Fowler DH, Luznik L, Nakamura R, O'Donnell PV, Perales MA, Pingali SR, Porter DL, Riches MR, Ringdén OT, Rocha V, Vij R, Weisdorf DJ, Champlin RE, Horowitz MM, Fuchs EJ, Eapen M (2015) Haploidentical transplant with posttransplant cyclophosphamide vs matched unrelated donor transplant for acute myeloid leukemia. Blood 126(8):1033–1040. https://doi.org/10.1182/blood-2015-04-639831
Kasamon YL, Bolaños-Meade J, Prince GT, Tsai HL, McCurdy SR, Kanakry JA, Rosner GL, Brodsky RA, Perica K, Smith BD, Gladstone DE, Swinnen LJ, Showel MM, Matsui WH, Huff CA, Borrello I, Pratz KW, McDevitt MA, Gojo I, Dezern AE, Shanbhag S, Levis MJ, Luznik L, Ambinder RF, Fuchs EJ, Jones RJ (2015) Outcomes of nonmyeloablative HLA-haploidentical blood or marrow transplantation with high-dose post-transplantation cyclophosphamide in older adults. J Clin Oncol 33(28):3152–3161. https://doi.org/10.1200/jco.2014.60.4777
McCurdy SR, Kanakry JA, Showel MM, Tsai HL, Bolaños-Meade J, Rosner GL, Kanakry CG, Perica K, Symons HJ, Brodsky RA, Gladstone DE, Huff CA, Pratz KW, Prince GT, Dezern AE, Gojo I, Matsui WH, Borrello I, McDevitt MA, Swinnen LJ, Smith BD, Levis MJ, Ambinder RF, Luznik L, Jones RJ, Fuchs EJ, Kasamon YL (2015) Risk-stratified outcomes of nonmyeloablative HLA-haploidentical BMT with high-dose posttransplantation cyclophosphamide. Blood 125(19):3024–3031. https://doi.org/10.1182/blood-2015-01-623991
Battipaglia G, Labopin M, Kröger N, Vitek A, Afanasyev B, Hilgendorf I, Schetelig J, Ganser A, Blaise D, Itälä-Remes M, Passweg JR, Bonifazi F, Finke J, Ruggeri A, Nagler A, Mohty M (2019) Posttransplant cyclophosphamide vs antithymocyte globulin in HLA-mismatched unrelated donor transplantation. Blood 134(11):892–899. https://doi.org/10.1182/blood.2019000487
Tang F, Xu Y, Chen H, Xu L, Zhang X, Wang Y, Liu Q, Wu D, Huang X (2019) Comparison of the clinical outcomes of hematologic malignancies after myeloablative haploidentical transplantation with G-CSF/ATG and posttransplant cyclophosphamide: results from the Chinese Bone Marrow Transplantation Registry Group (CBMTRG). Sci China Life Sci 63:571–581. https://doi.org/10.1007/s11427-019-9594-7
Funding
This work was supported by the Capital’s Funds for Health Improvement and Research (grant number 2018-4-4089), Peking University Medicine Fund of Fostering Young Scholars’ Scientific and Technological Innovation (grant number BMU2020PY007), National Key Research and Development Program of China (grant number 2017YFA0104500), the Key Program of the National Natural Science Foundation of China (grant number 81530046 and 81930004), the Foundation for Innovative Research Groups of the National Natural Science Foundation of China (grant number 81621001), the Science and Technology Project of Guangdong Province of China (grant number 2016B030230003), Peking University Clinical Scientist Program (BMU2019LCKXJ003), National Science and Technology Major Project (grant number 2017ZX09304021), and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 17 kb)
ESM 2
(DOCX 19 kb)
Fig S1
Supplementary figure 1. Toxicities of ruxolitinib in the 5mg BID group and >5mg BID group: (A) hematologic toxicities of aGVHD (5mg BID: n=13, >5mg BID: n=2); (B)infectious toxicities of aGVHD; (C) hematologic toxicities of cGVHD (5mg BID: n=14, >5mg BID: n=25; (D) infectious toxicities of cGVHD (TIF 288 kb)
Rights and permissions
About this article
Cite this article
Zhao, JY., Liu, SN., Xu, LP. et al. Ruxolitinib is an effective salvage treatment for multidrug-resistant graft-versus-host disease after haploidentical allogeneic hematopoietic stem cell transplantation without posttransplant cyclophosphamide. Ann Hematol 100, 169–180 (2021). https://doi.org/10.1007/s00277-020-04273-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04273-2