Abstract
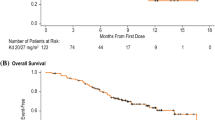
From April 2016, carfilzomib, in combination with lenalidomide and dexamethasone (KRD), became available for use in the daily practice in Italy for patients with relapsed or refractory multiple myeloma (RRMM). We performed a retrospective survey at 14 different institutions from Southern Italy in order to evaluate patient characteristics and treatment results from an unselected series of patients treated accordingly so far. One hundred and twenty-three consecutive patients were included, with a median of 2 previous lines of therapy (range 1–9) and a median age of 63 years (range 39–82). At the time of analysis, median number of courses administered is 11 (range 1–34), and all patients are evaluable for response. Overall response rate including complete remission, very good partial remission, and partial remission is 85%. After a median follow-up of 27 months, median overall and progression-free survival are 33 and 23 months, respectively. Sixty-three patients are alive and between them, 45 (37%) are in continuous remission. Sixty patients have died (49%), mainly from progressive disease. There were 6 treatment-related deaths (5% of the whole patient population). Overall, hematological and non-hematological toxicity were manageable, mostly on outpatient basis. Arterial hypertension has been observed in 43 cases (35%) but did not lead to treatment interruption. Our data demonstrate that in real life, KRD is highly effective and well tolerated in the majority of patients with RRMM.


Similar content being viewed by others
References
Jagannath S, Abonour R, Durie BGM, Gasparetto C, Hardin JW, Narang M, Terebelo HR, Toomey K, Wagner L, Srinivasan S, Kitali A, Yue L, Flick ED, Agarwal A, Rifkin RM (2018) Heterogeneity of second-line treatment for patients with multiple myeloma in the connect MM registry (2010-2016). Clin Lymphoma Myeloma Leuk 18(7):480–485
Anderson KC (2016) Progress and paradigms in multiple myeloma. Clin Cancer Res 22(22):5419–5427
Agarwal A, Chow E, Bhutani M, Voorhees PM, Friend R, Usmani SZ (2017) Practical considerations in managing relapsed multiple myeloma. Clin Lymphoma Myeloma Leuk 17(2):69–77
Mohty M, Terpos E, Mateos MV, Cavo M, Lejniece S, Beksac M, Bekadja MA, Legiec W, Dimopoulos M, Stankovic S, Durán MS, de Stefano V, Corso A, Kochkareva Y, Laane E, Berthou C, Salwender H, Masliak Z, Pečeliūnas V, Willenbacher W, Silva J, Louw V, Nemet D, Borbényi Z, Abadi U, Pedersen RS, Černelč P, Potamianou A, Couturier C, Feys C, Thoret-Bauchet F, Boccadoro M, Bekadja M, Hamladji RM, Ali HA, Hamdi S, Touhami H, Mansour NS, Willenbacher W, Linkesch W, Nemet D, Pedersen RS, Abildgaard N, Laane E, Hein M, Mohty M, Eveillard JR, Yamani A, Moreau P, Sanhes L, Lepeu G, Laribi K, Jourdan E, Fitoussi O, Allangba O, Fleury J, Escoffre M, Benramdane R, Cartron G, Dine G, Legouffe E, Harich HD, Illmer T, Dörfel S, Hannig CV, Koenigsmann M, Prange-Krex G, Salwender H, Tamm I, Zeller W, Maasberg M, Schlag R, Klausmann M, Uhlig J, Alkemper B, Schütz S, Tessen HW, Mohr B, Schmidt P, Heinrich B, Hebart H, Seipelt G, Zoeller T, Heits F, Müller-Naendrup C, Hansen R, Repp R, von Weikersthal LF, Schmits R, Heßling J, Krammer-Steiner B, Janzen V, Schauer M, Grüner MW, Kisro J, Denzlinger C, Freier W, Junghanss C, Görner M, Laichinger K, Ostermann H, Dürk H, Hess G, Reich G, Terpos E, Dimopoulos M, Matsouka P, Pouli A, Anagnostopoulos A, Masszi T, Borbényi Z, Ivanyi J, Szomor A, Abadi U, Nagler A, Magen H, Avivi I, Quitt M, Palumbo A, Boccadoro M, de Stefano V, Za T, Vallisa D, Foa R, Corso A, Bosi A, Vacca A, Lanza F, Palazzo G, Avvisati G, Cavo M, Ferrara F, Consoli U, Cantonetti M, Angelucci E, Califano C, di Raimondo F, Guarini A, Musso M, Pizzuti M, Giuliani N, Ardizzoia A, di Renzo N, Gaidano G, Gozzetti A, Pitini V, Farina G, Centurioni R, de Fabritiis P, Iuliano F, la Nasa G, la Verde G, Pane F, Recine U, la Targia M, Mineo G, Cangialosi C, Fagnani D, Federici A, Romano A, Specchia G, Storti S, Bongarzoni V, Bacigalupo A, Gobbi M, Latte G, Mannina D, Capalbo S, Lejniece S, Pečeliūnas V, Jurgutis M, Stankovic S, Legiec W, Woszczyk D, Hołojda J, Gornik S, Pluta A, Morawiec-Szymonik E, Kyrcz-Krzemien S, Homenda W, Grosicki S, Sulek K, Lange A, Kloczko J, Starzak-Gwozdz J, Hellmann A, Komarnicki M, Kuliczkowski K, Viveiros C, Gonçalves C, Esefyeva N, Kochkareva J, Kaplanov K, Volodicheva E, Laricheva E, Dergacheva V, Chukavina M, Volchenko N, Nazarova I, Anchukova L, Ovanesova E, Gritsenko T, Salogub G, Magomedova L, Kuznetsova I, Osyunikhina S, Serdyuk O, Karyagina E, Ivanova V, Černelč SP, Louw V, Coetzee C, Gunther K, Moodley D, Duran S, Gutiérrez AE, de Oteyza JP, Capote FJ, Casanova M, Sanchez JM, Rios-Herranz E, Ibañez-Garcia J, Herranz MJ, Hernandez B, Sanchez SS, Escalante F, Carnicero F, Lleonart JB, Gironella M, Martínez R, de la Guia AL, Palomera L, Iglesias R, Ramos FS, de la Serna J, Sanchez PG, Vidal JB, Mateos MV, Morfa MD, Beksac T–M, Vural F, Aydin Y, Unal A, Goker H, Bilgir O, Guvenc B, Turgut M, Ozet GG, Ali R, Masliak Z, Kyselyova M, Glushko N, Vybyrana R, Skrypnyk I, Tretyak N, Kharchevska T, Dyagil I, Popovs'ka T, Shimanskiy V, Lysa T, Oliynyk H, Vilchevskaya K, Kryachok I, Popovych Y, Romanyuk N, Yushchenko N, Kaplan P, Rekhtman G, Pylypenko H, Kozlov V, Mohty M, Terpos E, Mateos MV, Palumbo A, Drach J, Boccadoro M, Harousseau JL, Einsele H, Goldschmidt H, Facon T, Michalet M, Savchenko VG, de la Rubia J, Cook G, Mellqvist UH, Ludwig H (2018) Multiple myeloma treatment in real-world clinical practice: results of a prospective, multinational, noninterventional study. Clin Lymphoma Myeloma Leuk 18(10):e401–e419. https://doi.org/10.1016/j.clml.2018.06.018
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A, Hájek R, Rosiñol L, Siegel DS, Mihaylov GG, Goranova-Marinova V, Rajnics P, Suvorov A, Niesvizky R, Jakubowiak AJ, San-Miguel JF, Ludwig H, Wang M, Maisnar V, Minarik J, Bensinger WI, Mateos MV, Ben-Yehuda D, Kukreti V, Zojwalla N, Tonda ME, Yang X, Xing B, Moreau P, Palumbo A, ASPIRE Investigators (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372(2):142–152
Niesvizky R, Martin TG III, Bensinger WI et al (2013) Phase Ib dose-escalation study (PX-171-006) of carfilzomib, lenalido- mide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Clin Cancer Res 19:2248–2256
Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E, Huang M, Orlowski RZ, Niesvizky R (2013) Phase 2 dose-expansion study (PX-171- 006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood 122:3122–3128
Tzogani K, Camarero Jiménez J, Garcia I, Sancho-López A, Martin M, Moreau A, Demolis P, Salmonson T, Bergh J, Laane E, Ludwig H, Gisselbrecht C, Pignatti F (2017) The European medicines agency review of carfilzomib for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. Oncologist 22(11):1339–1346
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, Rosiñol L, Straub J, Suvorov A, Araujo C, Rimashevskaya E, Pika T, Gaidano G, Weisel K, Goranova-Marinova V, Schwarer A, Minuk L, Masszi T, Karamanesht I, Offidani M, Hungria V, Spencer A, Orlowski RZ, Gillenwater HH, Mohamed N, Feng S, Chng WJ, ENDEAVOR Investigators (2016) Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 17(1):27–38
Muchtar E, Gatt ME, Rouvio O, Ganzel C, Chubar E, Suriu C, Tadmor T, Shevetz O, Lavi N, Shochat T, Cohen YC, Avivi I, Raanani P, Magen H (2016) Efficacy and safety of salvage therapy using carfilzomib for relapsed or refractory multiple myeloma patients: a multicentre retrospective observational study. Br J Haematol 172(1):89–96
Rifkin RM, Medhekar E, Amirian SE et al (2019) A real-world comparative analysis of carfilzomib and other systemic multiple myeloma chemotherapies in a US community oncology setting. Ther Adv Hematol 10:1–10
Antonioli E, Staderini M, Nozzoli C et al (2017) “Real-life” experience of carfilzomib combined with lenalidomide and dexamethasone in relapsed/refractory multiple myeloma patients. Haematologica 102(s3):131–132
Barilà G, Meneghini V, Bonalumi A et al (2018) KRD treatment of relapsed/refractory multiple myeloma patients: a real life experience. Hemasphere 2(s1):978–979
Calafiore V, Martino E, Parisi M et al (2018) Efficacy, safety and tolerability of carfilzomib- lenalidomide-dexamethasone (KRD) regimen in RR/MM. Haematologica 103(s1):35–36
Guidotti F, Ferla V, Gregorini AI, Rossi FG, Pompa A (2018) Carfilzomib-lenalidomide-dexamethasone in relapsed/refractory multiple myeloma: a single centre real-life experience. Haematologica 103(s1):38
Terpos E, Caers J, Sohne M et al (2018) Real-world evidence of the use of carfilzomib among patients with relapsed multiple myeloma in Europe: an interim analysis for a prospective observational study. Hemasphere 2(s1):962
Dimopoulos MA, Roussou M, Gavriatopoulou M, Psimenou E, Ziogas D, Eleutherakis-Papaiakovou E, Fotiou D, Migkou M, Kanellias N, Panagiotidis I, Ntalianis A, Papadopoulou E, Stamatelopoulos K, Manios E, Pamboukas C, Kontogiannis S, Terpos E, Kastritis E (2017) Cardiac and renal complications of carfilzomib in patients with multiple myeloma. Blood Adv 1(7):449–454
Garderet L, Iacobelli S, Koster L, Goldschmidt H, Johansson JE, Bourhis JH, Krejci M, Leleu X, Potter M, Blaise D, Koenecke C, Peschel C, Radocha J, Metzner B, Lenain P, Schäfer-Eckart K, Pohlreich D, Grasso M, Caillot D, Einsele H, Ladetto M, Schönland S, Kröger N (2018) Outcome of salvage third autologous stem cell transplantation in multiple myeloma. Biol Blood Marrow Transplant 24(7):1372–1378
Hagen PA, Stiff P (2018) The role of salvage second autologous hematopoietic cell transplantation in relapsed multiple myeloma. Biol Blood Marrow Transplant 25:e98–e107. https://doi.org/10.1016/j.bbmt.2018.12.002
Dimopoulos M, Wang M, Maisnar V, Minarik J, Bensinger W, Mateos MV, Obreja M, Blaedel J, Moreau P (2018) Response and progression-free survival according to planned treatment duration in patients with relapsed multiple myeloma treated with carfilzomib, lenalidomide, and dexamethasone (KRd) versus lenalidomide and dexamethasone (Rd) in the phase III ASPIRE study. J Hematol Oncol 11(1):49. https://doi.org/10.1186/s13045-018-0583-7
Moreau P, Mateos MV, Berenson JR, Weisel K, Lazzaro A, Song K, Dimopoulos MA, Huang M, Zahlten-Kumeli A, Stewart AK (2018) Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): interim analysis results of a randomised, phase 3 study. Lancet Oncol 19(7):953–964
Bringhen S, Mina R, Cafro AM, Liberati AM, Spada S, Belotti A, Gaidano G, Patriarca F, Troia R, Fanin R, de Paoli L, Rossi G, Lombardo A, Bertazzoni P, Palumbo A, Sonneveld P, Boccadoro M (2018) Once-weekly carfilzomib, pomalidomide, and low-dose dexamethasone for relapsed/refractory myeloma: a phase I/II study. Leukemia 32(8):1803–1807
Dimopoulos MA, Oriol A, Nahi H, POLLUX Investigators et al (2016) Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 375(14):1319–1331
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Palmieri, S., Rocco, S., Vitagliano, O. et al. KRD (carfilzomib and lenalidomide plus dexamethasone) for the treatment of relapsed or refractory multiple myeloma in the real-life: a retrospective survey in 123 patients. Ann Hematol 99, 2903–2909 (2020). https://doi.org/10.1007/s00277-020-04158-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-020-04158-4