Abstract
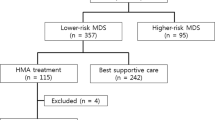
Predictive factors for initiating hypomethylating agents’ (HMAs) treatment and the survival benefit of HMAs for lower-risk myelodysplastic syndrome (LR-MDS) are still unknown. This study evaluated the factors affecting the use of HMAs and compared long-term outcomes between best supportive care (BSC) and HMA groups after matching baseline clinical factors. Data of 353 patients diagnosed with LR-MDS by International Prognostic Scoring System between October 1992 and July 2013 were retrospectively analyzed. HMAs were administered continuously until a clinical response or progression. HMAs were administered to 243 patients with median 45 days (range 0–7078 days) after diagnosis, while 110 patients were treated with BSC. HMAs were administered over a median of 5 cycles and overall response was achieved in 104 patients (42.8%). The cumulative incidence of HMA treatment increased in higher-risk groups by other risk scoring systems. Three-year overall survival (OS) rate was higher in BSC group (69.1%) than HMA responders (47.4%, p = 0.065) or HMA non-responders (46.3%, p = 0.005). Among 162 case-matched cohorts, 3-year OS rates were comparable between the BSC group (67.1%) and HMA responders (58.1%, p = 0.914), while that of HMA non-responder was low (32.2%, p < 0.001). In the case-matched cohorts, HMA non-responder were associated with inferior OS rate in the multivariate analysis (hazard ratio 3.01, p = 0.001). Higher-risk groups by other clinical risk scoring systems among IPSS lower-risk patients showed an increased incidence of using HMAs. The OS rate of HMA responders among case-matched cohorts showed an improved OS rate similar to the BSC group.

Similar content being viewed by others
References
Tefferi A, Vardiman JW (2009) Myelodysplastic syndromes. N Engl J Med 361(19):1872–1885
Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Sole F, Bennett JM, Bowen D, Fenaux P, Dreyfus F, Kantarjian H, Kuendgen A, Levis A, Malcovati L, Cazzola M, Cermak J, Fonatsch C, Le Beau MM, Slovak ML, Krieger O, Luebbert M, Maciejewski J, Magalhaes SM, Miyazaki Y, Pfeilstocker M, Sekeres M, Sperr WR, Stauder R, Tauro S, Valent P, Vallespi T, van de Loosdrecht AA, Germing U, Haase D (2012) Revised International Prognostic Scoring System for myelodysplastic syndromes. Blood 120(12):2454–2465
Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, Sanz M, Vallespi T, Hamblin T, Oscier D, Ohyashiki K, Toyama K, Aul C, Mufti G, Bennett J (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89(6):2079–2088
Mittelman M, Oster HS, Hoffman M, Neumann D (2010) The lower risk MDS patient at risk of rapid progression. Leuk Res 34(12):1551–1555
Greenberg PL, Attar E, Bennett JM, Bloomfield CD, De Castro CM, Deeg HJ, Foran JM, Gaensler K, Garcia-Manero G, Gore SD, Head D, Komrokji R, Maness LJ, Millenson M, Nimer SD, O’Donnell MR, Schroeder MA, Shami PJ, Stone RM, Thompson JE, Westervelt P, National Comprehensive Cancer N (2011) NCCN clinical practice guidelines in oncology: myelodysplastic syndromes. J Natl Compr Cancer Netw 9(1):30–56
Garcia-Manero G, Shan J, Faderl S, Cortes J, Ravandi F, Borthakur G, Wierda WG, Pierce S, Estey E, Liu J, Huang X, Kantarjian H (2008) A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia 22(3):538–543
Sohn SK, Moon JH, Lee YJ, Park SW, Kim JY (2016) Survey of experts on therapeutic policies and proposals for the optimal timing for allogeneic peripheral blood stem cell transplantation in transfusion-dependent patients with myelodysplastic syndrome-refractory anemia. Blood Res 51(1):44–49
Santini V (2016) Treatment of low-risk myelodysplastic syndromes. Hematol Am Soc Hematol Educ Program 2016(1):462–469
Montalban-Bravo G, Garcia-Manero G (2018) Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am J Hematol 93(1):129–147
Greenberg PL, Stone RM, Al-Kali A, Barta SK, Bejar R, Bennett JM, Carraway H, De Castro CM, Deeg HJ, DeZern AE, Fathi AT, Frankfurt O, Gaensler K, Garcia-Manero G, Griffiths EA, Head D, Horsfall R, Johnson RA, Juckett M, Klimek VM, Komrokji R, Kujawski LA, Maness LJ, O’Donnell MR, Pollyea DA, Shami PJ, Stein BL, Walker AR, Westervelt P, Zeidan A, Shead DA, Smith C (2017) Myelodysplastic syndromes, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw 15(1):60–87
Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR, International Vidaza High-Risk MDSSSG (2009) Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: a randomised, open-label, phase III study. Lancet Oncol 10(3):223–232
Kim YJ, Jang JH, Kwak JY, Lee JH, Kim HJ (2013) Use of azacitidine for myelodysplastic syndromes: controversial issues and practical recommendations. Blood Res 48(2):87–98
Kantarjian H, Issa JP, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, Klimek V, Slack J, de Castro C, Ravandi F, Helmer R 3rd, Shen L, Nimer SD, Leavitt R, Raza A, Saba H (2006) Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer 106(8):1794–1803
Komrokji R, Swern AS, Grinblatt D, Lyons RM, Tobiasson M, Silverman LR, Sayar H, Vij R, Fliss A, Tu N, Sugrue MM (2018) Azacitidine in lower-risk myelodysplastic syndromes: a meta-analysis of data from prospective studies. Oncologist 23(2):159–170
Lee JH, Kim YJ, Sohn SK, Yoon SS, Kim H, Cheong JW, Lee WS, Lee GW, Lim SN, Kim MK, Lee HS, Kim HJ, Hematology AMWPotKSo (2017) Benefits of hypomethylating therapy in IPSS lower-risk myelodysplastic syndrome patients: a retrospective multicenter case series study. Leuk Res 60:135–144
Giagounidis A (2017) Current treatment algorithm for the management of lower-risk MDS. Hematol Am Soc Hematol Educ Program 2017(1):453–459
Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, Pinto A, Beran M, de Witte TM, Stone RM, Mittelman M, Sanz GF, Gore SD, Schiffer CA, Kantarjian H (2006) Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108(2):419–425
Alessandrino EP, Della Porta MG, Bacigalupo A, Van Lint MT, Falda M, Onida F, Bernardi M, Iori AP, Rambaldi A, Cerretti R, Marenco P, Pioltelli P, Malcovati L, Pascutto C, Oneto R, Fanin R, Bosi A, Gruppo Italiano Trapianto di Midollo O (2008) WHO classification and WPSS predict posttransplantation outcome in patients with myelodysplastic syndrome: a study from the Gruppo Italiano Trapianto di Midollo Osseo (GITMO). Blood 112(3):895–902
Kantarjian H, O’Brien S, Ravandi F, Cortes J, Shan J, Bennett JM, List A, Fenaux P, Sanz G, Issa JP, Freireich EJ, Garcia-Manero G (2008) Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer 113(6):1351–1361
Falantes JF, Calderon C, Marquez Malaver FJ, Alonso D, Martin Noya A, Carrillo E, Martino ML, Montero I, Gonzalez J, Parody R, Espigado I, Perez-Simon JA (2013) Clinical prognostic factors for survival and risk of progression to acute myeloid leukemia in patients with myelodysplastic syndromes with < 10% marrow blasts and non-unfavorable cytogenetic categories. Clin Lymphoma Myeloma Leuk 13(2):144–152
Falantes J, Delgado RG, Calderon-Cabrera C, Marquez-Malaver FJ, Valcarcel D, de Miguel D, Bailen A, Bargay J, Bernal T, Gonzalez-Porras JR, Tormo M, Ramos F, Andreu R, Xicoy B, Nomdedeu B, Brunet S, Sanchez J, Jurado AF, Bonanad S, Perez-Simon JA, Sanz G, Spanish Group of Myelodysplastic S (2015) Multivariable time-dependent analysis of the impact of azacitidine in patients with lower-risk myelodysplastic syndrome and unfavorable specific lower-risk score. Leuk Res 39(1):52–57
Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF (2002) Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol 20(10):2429–2440
Malcovati L, Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, Passamonti F, Arcaini L, Maffioli M, Bernasconi P, Lazzarino M, Cazzola M (2005) Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol 23(30):7594–7603
Lamarque M, Raynaud S, Itzykson R, Thepot S, Quesnel B, Dreyfus F, Rauzy OB, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Visanica S, Stamatoullas A, Fenaux P, Ades L (2012) The revised IPSS is a powerful tool to evaluate the outcome of MDS patients treated with azacitidine: the GFM experience. Blood 120(25):5084–5085
Gangat N, Mudireddy M, Lasho TL, Finke CM, Nicolosi M, Szuber N, Patnaik MM, Pardanani A, Hanson CA, Ketterling RP, Tefferi A (2018) Mutations and prognosis in myelodysplastic syndromes: karyotype-adjusted analysis of targeted sequencing in 300 consecutive cases and development of a genetic risk model. Am J Hematol 93(5):691–697
Gangat N, Patnaik MM, Tefferi A (2016) Myelodysplastic syndromes: contemporary review and how we treat. Am J Hematol 91(1):76–89
Migdady Y, Barnard J, Al Ali N, Steensma DP, DeZern A, Roboz G, Garcia-Manero G, Sekeres MA, Komrokji RS (2018) Clinical outcomes with ring sideroblasts and SF3B1 mutations in myelodysplastic syndromes: MDS clinical research consortium analysis. Clin Lymphoma Myeloma Leuk
Bejar R, Stevenson KE, Caughey BA, Abdel-Wahab O, Steensma DP, Galili N, Raza A, Kantarjian H, Levine RL, Neuberg D, Garcia-Manero G, Ebert BL (2012) Validation of a prognostic model and the impact of mutations in patients with lower-risk myelodysplastic syndromes. J Clin Oncol 30(27):3376–3382
Anderson JR, Cain KC, Gelber RD (1983) Analysis of survival by tumor response. J Clin Oncol 1(11):710–719
Davidoff AJ, Weiss SR, Baer MR, Ke X, Hendrick F, Zeidan A, Gore SD (2013) Patterns of erythropoiesis-stimulating agent use among Medicare beneficiaries with myelodysplastic syndromes and consistency with clinical guidelines. Leuk Res 37(6):675–680
Kelaidi C, Park S, Sapena R, Beyne-Rauzy O, Coiteux V, Vey N, Stamatoullas A, Choufi B, Delaunay J, Gourin MP, Cheze S, Ravoet C, Ferrant A, Escoffre-Barbe M, Aljassem L, Raffoux E, Itzykson R, Ades L, Dreyfus F, Fenaux P (2013) Long-term outcome of anemic lower-risk myelodysplastic syndromes without 5q deletion refractory to or relapsing after erythropoiesis-stimulating agents. Leukemia 27(6):1283–1290
Jadersten M, Montgomery SM, Dybedal I, Porwit-MacDonald A, Hellstrom-Lindberg E (2005) Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood 106(3):803–811
Lyons RM, Cosgriff TM, Modi SS, Gersh RH, Hainsworth JD, Cohn AL, McIntyre HJ, Fernando IJ, Backstrom JT, Beach CL (2009) Hematologic response to three alternative dosing schedules of azacitidine in patients with myelodysplastic syndromes. J Clin Oncol 27(11):1850–1856
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the Institutional Review Board of the each participating centers. All procedures performed in studies involving human participants were in accordance with the ethical standards of institutional and/or national research committees and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was not required for this type of study.
Electronic supplementary material
ESM 1
(DOCX 350 kb)
Rights and permissions
About this article
Cite this article
Baek, D.W., Lee, Y.J., Kim, H. et al. Response to hypomethylating agents improves long-term outcomes for lower-risk patients with myelodysplastic syndrome in case-matched cohorts. Ann Hematol 97, 2309–2317 (2018). https://doi.org/10.1007/s00277-018-3458-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3458-7