Abstract
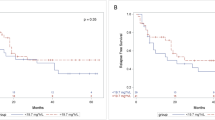
Busulfan (Bu) is an alkylating agent widely used in conditioning regimes prior to stem cell transplantation (SCT), most commonly in combination with cyclophosphamide (Bu-cy) or fludarabine (Bu-flu) as myeloablative conditioning prior to allograft or with high-dose melphalan (Bu-mel) prior to autologous SCT. Despite many decades of Bu use, initially orally but now intravenously (IV), a paucity of pharmacokinetic (PK) and pharmacodynamic (PD) data exists to inform evidence-based guidelines as how best to balance the efficacy and toxicity of this agent. This is a non-randomized retrospective real-world study at three hospitals investigating the role of PK guidance in dosing Bu in the setting of IV Bu-mel autologous SCT. The primary intent was to examine how effectively PK assessment could be used to achieve a desirable drug exposure and to evaluate factors, particularly, age, sex, actual weight, body mass index (BMI), body surface area (BSA), disease, number of prior treatments, renal function, and the use of concomitant medications that may influence Bu exposure. All patients underwent PK analysis on a test dose of Bu (1.6 mg/kg, i.e., 50% of the first dose) on D-7 and subsequently received a second 1.6 mg/kg dose on D-6. Bu dose was calculated using actual body weight (ABW) if patients were less than ideal body weight (IBW), or adjusted ideal body weight (AIBW) if ABW was greater than IBW. Thereafter, at the discretion of the investigator, the group was divided into two; a weight-based cohort at two hospitals and a PK-guided cohort at the third hospital. Thirty-seven patients received PK-adjusted dosing guided by the results of the initial PK results, targeting a specific Bu exposure expressed as the area-under-the-concentration-versus-time curve (AUC) of between 4000 and 5000 μmol min/day (median 4800). The remaining 27 patients received unadjusted weight-based doses with a further three doses of 3.2 mg/kg of Bu infused over 180 min (D-5 to − 3) irrespective of their initial PK results. For the purposes of the analysis, we selected a target AUC of 4800 μmol min/day in this group, equivalent to the median targeted AUC in the PK-adjusted group. All patients subsequently had repeated PK analysis on D-5 after receiving their “therapeutic” Bu dose. Mel (140 mg/m2 or 100 mg/m2) IV was given on D-2. Sixty-four adult patients were enrolled. Patients who received PK-guided Bu dosing received a higher median Bu dose than the unadjusted weight-based cohort (3.5 mg/kg vs 3.2 mg/kg respectively, p = 0.007). Eighty-one percent (30/37) of patients in the PK-guided group achieved their target AUC (± 15%) compared with 56% (15/27) in the weight-based cohort (p = 0.027). The respective median AUCs of 5064 μmol min/day (range 3639–6157 μmol min/day) and 4854 μmol min/day (range 3251–6305 μmol min/day) were not significantly different (p = 0.16). Multivariate analysis identified ABW as the only independent variable that affected the relationship between Bu dosing and exposure (p = 0.02) with heavier patients achieving lower than anticipated AUCs for the dose they received. On D-5, within the weight-based cohort, the mean AUCs were 12% higher than anticipated based on initial D-7 PK. No correlation between AUC and grade 3–4 transplant-related toxicities were observed, although only three patients had AUCs > 6000 μmol min/day. These results suggest that PK-directed Bu dosing may be of benefit in achieving a target level of drug exposure, with larger studies needed to determine the clinical significance of this strategy.


Similar content being viewed by others
References
Nivison-Smith I, Bradstock KF, Dodds AJ, Hawkins PA, Ma DDF, Moore JJ, Simpson JM, Szer J (2007) Hematopoietic stem cell transplantation in Australia and New Zealand, 1992-2004. Biol Blood Marrow Transplant 13:905–912
Majhail NS, Farnia SH, Carpenter PA, Champlin RE, Crawford S, Marks DI, Omel JL, Orchard PJ, Palmer J, Saber W, Savani BN, Veys PA, Bredeson CN, Giralt SA, LeMaistre CF (2015) Indications for autologous and allogeneic hematopoietic cell transplantation: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 21:1863–1869
Nieto Y, Popat U, Anderlini P, Valdez B, Andersson B, Liu P, Hosing C, Shpall EJ, Alousi A, Kebriaei P, Qazilbash M, Parmar S, Bashir Q, Shah N, Khouri I, Rondon G, Champlin R, Jones RB (2013) Autologous stem cell transplantation for refractory or poor-risk relapsed Hodgkin’s lymphoma: effect of the specific high-dose chemotherapy regimen on outcome. Biol Blood Marrow Transplant 19:410–417
Shah N, Callander N, Ganguly S, Gul Z, Hamadani M, Costa L, Sengsayadeth S, Abidi M, Hari P, Mohty M, Chen YB, Koreth J, Landau H, Lazarus H, Leather H, Majhail N, Nath R, Osman K, Perales MA, Schriber J, Shaughnessy P, Vesole D, Vij R, Wingard J, Giralt S, Savani BN, American Society for Blood and Marrow Transplantation (2015) Hematopoietic stem cell transplantation for multiple myeloma: guidelines from the American Society for Blood and Marrow Transplantation. Biol Blood Marrow Transplant 21:1155–1166
Giralt S et al (2015) American Society of Blood and Marrow Transplantation, European Society of Blood and Marrow Transplantation, Blood and Marrow Transplant Clinical Trials Network, and International Myeloma Working Group Consensus Conference on Salvage Hematopoietic Cell Transplantation in Patients with Relapsed Multiple Myeloma. Biol Blood Marrow Transplant 21:2039–2051
Nath CE, Shaw PJ, Trotman J, Zeng L, Duffull SB, Hegarty G, McLachlan AJ, Gurney H, Kerridge I, Kwan YL, Presgrave P, Tiley C, Joshua D, Earl J (2010) Population pharmacokinetics of melphalan in patients with multiple myeloma undergoing high dose therapy. Br J Clin Pharmacol 69:484–497
Lill M, Costa LJ, Yeh RF, Lim S, Stuart R, Waller EK, Shore T, Craig M, Freytes CO, Shea TC, Rodriguez TE, Flinn IW, Comeau T, Yeager AM, Pulsipher MA, Bence-Bruckler I, Laneuville P, Bierman PJ, Chen AI, Yu LH, Patil S, Sun Y, Armstrong E, Smith A, Elekes A, Kato K, Vaughan W (2013) Pharmacokinetic-directed dose adjustment is essential for intravenous busulfan exposure optimization: findings from a multi-center phase II study of autologous hematopoietic stem cell transplantation for lymphoma in North America. Biol Blood Marrow Transplant 19:S132
Flowers CR et al (2016) Efficacy of pharmacokinetics-directed busulfan, cyclophosphamide, and etoposide conditioning and autologous stem cell transplantation for lymphoma: comparison of a multicenter phase II study and CIBMTR outcomes. Biol Blood Marrow Transplant:1–9. https://doi.org/10.1016/j.bbmt.2016.03.018
Russell JA, Kangarloo SB (2008) Therapeutic drug monitoring of busulfan in transplantation. Curr Pharm Des 14:1936–1949
Slattery JT, Risler LJ (1998) Therapeutic monitoring of busulfan in hematopoietic stem cell transplantation. Ther Drug Monit 20:543–549
Gibbs JP, Gooley T, Corneau B, Murray G, Stewart P, Appelbaum FR, Slattery JT (1999) The impact of obesity and disease on busulfan oral clearance in adults. Blood 93:4436–4440
Wang Y, Kato K, le Gallo C, Armstrong E, Rock E, Wang X (2015) Dosing algorithm revisit for busulfan following IV infusion. Cancer Chemother Pharmacol 75:505–512
Kebriaei P et al (2011) Intravenous busulfan plus melphalan is a highly effective, well-tolerated preparative regimen for autologous stem cell transplantation in patients with advanced lymphoid malignancies. Biol Blood Marrow Transplant 17:412–420
Bartelink IH, Bredius RGM, Belitser SV, Suttorp MM, Bierings M, Knibbe CAJ, Egeler M, Lankester AC, Egberts ACG, Zwaveling J, Boelens JJ (2009) Association between busulfan exposure and outcome in children receiving intravenous busulfan before hematologic stem cell transplantation. Biol Blood Marrow Transplant 15:231–241
Ellen Nath C, John Shaw P (2007) Busulphan in blood and marrow transplantation: dose, route, frequency and role of therapeutic drug monitoring. Curr Clin Pharmacol 2:75–91
Clemmons AB, Evans S, DeRemer DL, Awan FT (2015) Busulfan dosing (Q6 or Q24) with adjusted or actual body weight, does it matter? J Oncol Pharm Pract 21:425–432
McCune JS, Gibbs JP, Slattery DJT (2000) Plasma concentration monitoring of busulfan. Clin Pharmacokinet 39:155–165
Andersson BS, Thall PF, Madden T, Couriel D, Wang X, Tran HT, Anderlini P, de Lima M, Gajewski J, Champlin RE (2002) Busulfan systemic exposure relative to regimen-related toxicity and acute graft-versus-host disease: defining a therapeutic window for i.V. BuCy2 in chronic myelogenous leukemia. Biol Blood Marrow Transplant 8:477–485
Ljungman P, Hassan M, Bekassy AN (1997) High busulfan concentrations are associated with increased transplant-related mortality in allogeneic bone marrow transplant patients. Bone Marrow Transplant 20:909–913
Slattery JT, Clift RA, Buckner CD, Radich J, Storer B, Bensinger WI, Soll E, Anasetti C, Bowden R, Bryant E, Chauncey T, Deeg HJ, Doney KC, Flowers M, Gooley T, Hansen JA, Martin PJ, McDonald G, Nash R, Petersdorf EW, Sanders JE, Schoch G, Stewart P, Storb R, Sullivan KM, Thomas ED, Witherspoon RP, Appelbaum FR (1997) Marrow transplantation for chronic myeloid leukemia: the influence of plasma busulfan levels on the outcome of transplantation. Blood 89:3055–3060
Slattery JT, Sanders JE, Buckner CD, Schaffer RL, Lambert KW, Langer FP, Anasetti C, Bensinger WI, Fisher LD, Appelbaum FR (1995) Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant 16:31–42
Russell JA, Kangarloo SB, Williamson T, Chaudhry MA, Savoie ML, Turner AR, Larratt L, Storek J, Bahlis NJ, Shafey M, Brown CB, Yang M, Geddes M, Zacarias N, Yue P, Duggan P, Stewart DA, Daly A (2013) Establishing a target exposure for once-daily intravenous busulfan given with fludarabine and thymoglobulin before allogeneic transplantation. Biol Blood Marrow Transplant 19:1381–1386
Bartelink IH, Lalmohamed A, van Reij EML, Dvorak CC, Savic RM, Zwaveling J, Bredius RGM, Egberts ACG, Bierings M, Kletzel M, Shaw PJ, Nath CE, Hempel G, Ansari M, Krajinovic M, Théorêt Y, Duval M, Keizer RJ, Bittencourt H, Hassan M, Güngör T, Wynn RF, Veys P, Cuvelier GDE, Marktel S, Chiesa R, Cowan MJ, Slatter MA, Stricherz MK, Jennissen C, Long-Boyle JR, Boelens JJ (2016) Association of busulfan exposure with survival and toxicity after haemopoietic cell transplantation in children and young adults: a multicentre, retrospective cohort analysis. Lancet Haematol 3:e526–e536
Weil E, Zook F, Oxencis C, Canadeo A, Urmanski A, Waggoner M, Eastwood D, Pasquini M, Hamadani M, Hari P (2017) Evaluation of the pharmacokinetics and efficacy of a busulfan test dose in adult patients undergoing myeloablative hematopoietic cell transplantation. Biol Blood Marrow Transplant 23:952–957
Patel JN, Papachristos A (2016) Personalizing chemotherapy dosing using pharmacological methods. Cancer Chemother Pharmacol 76:879–896
Nath CE, Trotman J, Tiley C, Presgrave P, Joshua D, Kerridge I, Kwan YL, Gurney H, McLachlan AJ, Earl JW, Nivison-Smith I, Zeng L, Shaw PJ (2016) High melphalan exposure is associated with improved overall survival in myeloma patients receiving high dose melphalan and autologous transplantation. Br J Clin Pharmacol 82:149–159
Nguyen L, Leger F, Lennon S, Puozzo C (2006) Intravenous busulfan in adults prior to haematopoietic stem cell transplantation: a population pharmacokinetic study. Cancer Chemother Pharmacol 57:191–198
National Institutes of Health, National Heart, Lung, and Blood Institute. Classification of Overweight and Obesity by BMI, Waist Circumference, and Associated Disease Risks. Available at: https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmi_dis.htm. Accessed: 6 August 2017
Hassan M, Ehrsson H (1983) Gas chromatographic determination of busulfan in plasma with electron-capture detection. J Chromatogr 277:374–380
Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47:207–214
Kangarloo SB et al (2012) Development and validation of a test dose strategy for once-daily i.v. busulfan: importance of fixed infusion rate dosing. Biol Blood Marrow Transplant 18:295–301
Tse JC, Brown B, Moore TB (2012) Pharmacokinetic and pharmcodynamic monitoring of once daily intravenous busulfan in pediatric patients receiving hematopoietic stem cells transplant: a single Instutution experience. Biol Blood Marrow Transplant 18:S356
Tse WT et al (2009) Age-dependent pharmacokinetic profile of single daily dose i.v. busulfan in children undergoing reduced-intensity conditioning stem cell transplant. Bone Marrow Transplant 44:145–156
Beri R et al (2010) Reliability of a pretransplant i.v. BU test dose performed 2 weeks before myeloablative FluBu conditioning regimen. Bone Marrow Transplant 45:249–253
Diestelhorst C, Boos J, McCune JS, Russell J, Kangarloo SB, Hempel G (2013) Physiologically based pharmacokinetic modelling of busulfan: a new approach to describe and predict the pharmacokinetics in adults. Cancer Chemother Pharmacol 72:991–1000
de Lima M (2004) Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood 104:857–864
Krivoy N, Hoffer E, Lurie Y (2008) Busulfan use in hematopoietic stem cell transplantation: pharmacology, dose adjustment, safety and efficacy in adults and children. Curr Drug Saf 3:60–66
Hassan M, Ljungman P, Bolme P, Ringdén O, Syrůcková Z, Békàssy A, Starý J, Wallin I, Kållberg N (1994) Busulfan bioavailability. Blood 84:2144–2150
Perkins J, Fields T, Kim J, Fernandez H, Perez L, Ayala E, Kharfan-Dabaja M, Tomblyn M, Sullivan D, Anasetti C (2011) Maximally tolerated busulfan area under the concentration-time curve (AUC) in combination with fludarabine as conditioning prior to allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 17:S302
Nath CE, Earl JW, Pati N, Stephen K, Shaw PJ (2008) Variability in the pharmacokinetics of intravenous busulphan given as a single daily dose to paediatric blood or marrow transplant recipients. Br J Clin Pharmacol 66:50–59
Krivoy N, Zuckerman T, Elkin H, Froymovich L, M. Rowe J, Efrati E (2012) Pharmacokinetic and pharmacogenetic analysis of oral busulfan in stem cell transplantation: prediction of poor drug metabolism to prevent drug toxicity. CDS 7:211–217
Ansari M et al (2013) Glutathione S-transferase gene variations influence BU pharmacokinetics and outcome of hematopoietic SCT in pediatric patients. Bone Marrow Transplant 48:939–946
Ansari M et al (2015) Influence of glutathione S-transferase gene polymorphisms on busulfan pharmacokinetics and outcome of hematopoietic stem-cell transplantation in thalassemia pediatric patients. Bone Marrow Transplant 51:377–383
Schuler U, Schroer S, Kühnle A, Blanz J, Mewes K, Kumbier I, Proksch B, Zeller KP, Ehninger G (1994) Busulfan pharmacokinetics in bone marrow transplant patients: is drug monitoring warranted? Bone Marrow Transplant 14:759–765
Booth BP et al (2013) Population pharmacokinetic-based dosing of intravenous busulfan in pediatric patients. J Clin Pharmacol 47:101–111
Bouligand J, Boland I, Valteau-Couanet D, Deroussent A, Kalifa C, Hartmann O, Vassal G (2003) In children and adolescents, the pharmacodynamics of high-dose busulfan is dependent on the second alkylating agent used in the combined regimen (melphalan or thiotepa). Bone Marrow Transplant 32:979–986
Diestelhorst C, Boos J, McCune JS, Russell J, Kangarloo SB, Hempel G (2014) Predictive performance of a physiologically based pharmacokinetic model of busulfan in children. Pediatr Hematol Oncol 31:731–742
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Willcox, A., Wong, E., Nath, C. et al. The pharmacokinetics and pharmacodynamics of busulfan when combined with melphalan as conditioning in adult autologous stem cell transplant recipients. Ann Hematol 97, 2509–2518 (2018). https://doi.org/10.1007/s00277-018-3447-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3447-x