Abstract
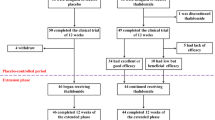
To investigate the efficacy and safety of thalidomide in patients with thalassemia intermedia (TI). Patients with a confirmed diagnosis of TI who met the trial criteria and signed consent forms were prescribed oral thalidomide 50 mg qn for 3 months from February 2017. Complete blood counts, Hb analysis, and liver and kidney functions were monitored monthly during treatment and any differences were compared before and after treatment. Patients with Hb increments > 2.0 g/dL were termed main responders (MaR), and those with Hb increments between 1.0 and 2.0 g/dL as minor responders (MiR), otherwise they were termed non-responders. Relevance analysis was performed to explore parameters predicting Hb increments after treatment. Adverse effects during treatment were carefully recorded. The overall response rate (ORR = MaR + MiR) and MaR rates were 78.6 and 50% after 1 month of treatment, respectively, and 85.7 and 71.4% after 3 months treatment. At the end of the treatment period, Hb and HbF increased by 2.5 ± 1.8 g/dL and 2.5 ± 1.6 g/dL, while bilirubin, lactate dehydrogenase, and the nucleated red blood cell count (NRBC) were significantly decreased, while the reticulocyte count significantly increased. Correlation analysis showed that the Hb increments correlated significantly with the ratio of HbF before treatment (r = 0.683, P = 0.007) rather than age, Hb, reticulocyte count, and NRBC before treatment. Adverse events during treatment were mild, and drug reduction or withdrawal from the trial was not required. Thalidomide had rapid and significant effects in patients with TI, and also, it is safe and convenient. But larger scale clinical trials will be required to confirm our conclusions. Trial Registration: NCT02995707, https://www.clinicaltrials.gov/ct2/show/NCT03184844?term=thalidomide+thalassemia&rank=1.


Similar content being viewed by others
References
Origa R (2016) β-Thalassemia. Genet Med
Padate B, Bain BJ, de la Fuente J (2011) Ineffective hemopoietic in beta thalassemia major visualised. Am J Hematol 86:372. https://doi.org/10.1002/ajh.21953
Choudhry VP (2017) Thalassemia minor and major: current management. Indian J Pediatr 84:607–611. https://doi.org/10.1007/s12098-017-2325-1
Camaschella C, Cappellini MD (1995) Thalassemia intermedia. Haematologica 80:58–68
Vichinsky E (2016) Non-transfusion-dependent thalassemia and thalassemia intermedia: epidemiology, complications, and management. Curr Med Res Opin 32:191–204. https://doi.org/10.1185/03007995.2015.1110128
Kosaryan M, Zafari M, Alipur A, Hedayatizadeh-Omran A (2014) The effect and side effect of hydroxyurea therapy on patients with β-thalassemia: a systematic review to December 2012. Hemoglobin 38:262–271
Voskaridou E, Christoulas D, Bilalis A, Plata E, Varvagiannis K, Stamatopoulos G, Sinopoulou K, Balassopoulou A, Loukopoulos D, Terpos E (2010) The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (LaSHS). Blood 115:2354–2363
Foong WC, Ho JJ, Loh CK, Viprakasit V (2016) Hydroxyurea for reducing blood transfusion in non-transfusion dependent beta thalassaemias. Cochrane Database Syst Rev 10:CD011579. https://doi.org/10.1002/14651858.CD011579.pub2
Olivieri NF, Saunthararajah Y, Thayalasuthan V, Kwiatkowski J, Ware RE, Kuypers FA, Kim HY, Trachtenberg FL, Vichinsky EP (2011) A pilot study of subcutaneous decitabine in beta-thalassemia intermedia. Blood 118:2708–2711
Maccio A, Madeddu C, Chessa P, Mantovani G, Galanello R (2009) Use of erythropoiesis stimulating agents for the treatment of anaemia and related fatigue in a pregnant woman with HbH disease. Br J Haematol 146:335–337. https://doi.org/10.1111/j.1365-2141.2009.07755.x
Aguilar-Lopez LB, Delgado-Lamas JL, Rubio-Jurado B, Perea FJ, Ibarra B (2008) Thalidomide therapy in a patient with thalassemia major. Blood Cells Mol Dis 41:136–137. https://doi.org/10.1016/j.bcmd.2008.03.001
Fozza C, Pardini S, Giannico DB, Targhetta C, Di TAA, Dessalvi P, Angelucci E, Dore F (2015) Dramatic erythroid response to low-dose thalidomide in two patients with transfusion independent thalassemia and severe post-transfusional alloimmune hemolysis. Am J Hematol 90:E141. https://doi.org/10.1002/ajh.24030
Ricchi P, Costantini S, Spasiano A, De Dominicis G, Di MT, Cinque P, Ammirabile M, Marsella M, Filosa A (2016) The long-term and extensive efficacy of low dose thalidomide in a case of an untransfusable patient with non-transfusion-dependent thalassemia. Blood Cells Mol Dis 57:97–99. https://doi.org/10.1016/j.bcmd.2016.01.003
Chen J, Zhu W, Cai N, Bu S, Li J, Huang L (2017) Thalidomide induces haematologic responses in patients with β-thalassaemia. Eur J Haematol 99:437–441
Li Y, Ren Q, Zhou Y, Li P, Lin W, Yin X (2018) Thalidomide has a significant effect in patients with thalassemia intermedia. Hematology 23:50–54. https://doi.org/10.1080/10245332.2017.1354427
Fang S, Wu Z, Zhang X, Liu Y, Wang W, Chai L, Cai H, Yi J, Wang L, Chen Y, Lv X, Huang Y, Wang R, Chen P (2007) Clinical observation on YiSuiShengXueGranule on treating 156 patients with beta-thalassemia major and the molecular mechanism study. Biol Pharm Bull 30:2084–2087
Koren A, Levin C, Dgany O, Kransnov T, Elhasid R, Zalman L, Palmor H, Tamary H (2008) Response to hydroxyurea therapy in beta-thalassemia. Am J Hematol 83:366–370
Bohara VV, Ray S, Chakrabarti P, Ray SS, Nath UK, Chaudhuri U (2014) Optimizing the dose of hydroxyurea therapy for patients with β-thalassemia intermedia (Hb E-β-thalassemia): a single center study from Eastern India. Hemoglobin 38:44–48
El-Beshlawy A, El-Ghamrawy M, EL-Ela MA, Said F, Adolf S, Abdel-Razek AR, Magdy RI, Abdel-Salam A (2014) Response to hydroxycarbamide in pediatric β-thalassemia intermedia: 8 years’ follow-up in Egypt. Ann Hematol 93:2045–2050
Karimi M, Haghpanah S, Farhadi A, Yavarian M (2012) Genotype-phenotype relationship of patients with β-thalassemia taking hydroxyurea: a 13-year experience in Iran. Int J Hematol 95:51–56
Ch A, Alimirzoyeva Z, Hasanova M, Mammadova T, Shirinova A (2016) Clinical application of recombinant erythropoietin in beta-thalassaemia intermedia. Georgian Med News 255:86–92
Jalali FMA, Dehghani FA, Hajizamani S, Mossahebi-Mohammadi M, Yaghooti H, Saki N (2016) Thalidomide is more efficient than sodium butyrate in enhancing GATA-1 and EKLF gene expression in erythroid progenitors derived from HSCs with β-globin gene mutation. Int J Hematol Oncol Stem Cell Res 10:37–41
Aerbajinai W, Zhu J, Gao Z, Chin K, Rodgers GP (2007) Thalidomide induces gamma-globin gene expression through increased reactive oxygen species-mediated p38 MAPK signaling and histone H4 acetylation in adult erythropoiesis. Blood 110:2864–2871. https://doi.org/10.1182/blood-2007-01-065201
Dixit A, Chatterjee TC, Mishra P, Choudhry DR, Mahapatra M, Tyagi S, Kabra M, Saxena R, Choudhry VP (2005) Hydroxyurea in thalassemia intermedia—a promising therapy. Ann Hematol 84:441–446
Danise P, Amendola G, Di CR, Cillari E, Gioia M, Di PA, Avino D, Rigano P, Maggio A (2009) Nucleated red blood cells and soluble transferrin receptor in thalassemiasyndromes: relationship with global and ineffective erythropoiesis. Clin Chem Lab Med 47:1539–1542
Motovali-Bashi M, Ghasemi T (2015) Role of XmnIgG polymorphism in hydroxyurea treatment and fetal hemoglobin level at Isfahanian intermediate β-thalassemia patients. Iran Biomed J 19:177–182
Elalfy MS, El SNH, Kamal TM, Aly NH (2017) Klf10 gene, a secondary modifier and a pharmacogenomic biomarker of hydroxyurea treatment among patients with hemoglobinopathies. J Pediatr Hematol Oncol 39:e155-155e162. https://doi.org/10.1097/MPH.0000000000000762, e162
Funding
This study was supported by projects from the National Natural Science Foundation of China (31201103) and the Natural Science Foundation of Guangxi, China (2015GXNSFAA139164 and 2014GXNSFBA118187).
Author information
Authors and Affiliations
Contributions
Xiao-Lin Yin designed the study; Li Wang, Yong-Sheng Chen, and Ya-Li Zhou presented the patients; Ping-Ping Li and Yan-Ni Ma performed the laboratory analyses; Quan Ren wrote the first draft of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Our study was approved by the medical ethics committee of the 303rd Hospital of the People’s Liberation Army and registered at ClinicalTrials.gov, registration number: NCT02995707. Based on the Declaration of Helsinki, written informed consent was obtained from all patients included in the trial.
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Ren, Q., Zhou, YL., Wang, L. et al. Clinical trial on the effects of thalidomide on hemoglobin synthesis in patients with moderate thalassemia intermedia. Ann Hematol 97, 1933–1939 (2018). https://doi.org/10.1007/s00277-018-3395-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-018-3395-5