Abstract
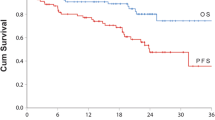
We sought to evaluate the activity and safety of carfilzomib-/ixazomib-containing combinations for patients with relapsed/refractory multiple myeloma (RRMM). We searched published reports including carfilzomib-/ixazomib-containing combinations for RRMM. Finally, we identified 11 prospective studies covering 2845 relapsed/refractory patients. Carfilzomib- and ixazomib-containing combinations respectively resulted in an impressive overall response rate (ORR 77 vs. 64%, P = 0.14), very good partial response or better (≥ VGPR 48 vs. 21%, P = 0.001), complete response or better (≥ CR 14 vs. 7%, P = 0.23), and clinical benefit rate (CBR 84 vs. 59%, P = 0.0002). Subgroup analysis showed that the carfilzomib (CFZ) +lenalidomide (LEN) + dexamethasone (DEX) triplet regimen resulted into similar response outcomes to those from CFZ + DEX doublet regimen in ORR (77 vs. 78%, P = 0.91), ≥VGPR (50 vs. 53%, P = 0.84), and ≥ CR (13 vs. 12%, P = 0.96) analysis in these previously heavily pretreated population. And, there were no statistically significant differences between IXA + LEN + DEX triplet regimen and CFZ + LEN + DEX triplet regimen in ORR (85 vs. 78%, P = 0.55), ≥ VGPR (37 vs. 53%, P = 0.19), and ≥ CR (18 vs. 12%, P = 0.70) analysis. There were favorable trend towards proteasome inhibitors (PIs) + IMiDs + DEX in comparison with PIs + alkylating agent + Dex in ORR (79 vs 49%, P < 0.00001), ≥ VGPR analysis (36 vs. 16%, P = 0.008), and ≥ CR (16 vs. 3%, P < 0.00001). Compared with current standard chemotherapy, carfilzomib containing combinations clearly improved overall survival (HR, 0.79; P = 0.01), progression free survival (HR, 0.61; P = 0.0001). Carfilzomib-/ixazomib-containing combinations produced clinical benefit for patients with R/RMM. PIs + IMiDs + DEX triplet regimens could be good options for such relapsed/refractory patients.




Similar content being viewed by others
References
Kumar SK, Rajkumar SV, Dispenzieri A, Lacy MQ, Hayman SR, Buadi FK et al (2008) Improved survival in multiple myeloma and the impact of novel therapies. Blood 111(5):2516–2520
Kumar SK, Lee JH, Lahuerta JJ, Morgan G, Richardson PG, Crowley J et al (2012) Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 26(1):149–157
Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV et al (2004) Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc 79(7):867–874
Berdeja JG, Hart LL, Mace JR, Arrowsmith ER, Essell JH, Owera RS et al (2015) Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica 100(5):670–676
Berenson JR, Cartmell A, Bessudo A, Lyons RM, Harb W, Tzachanis D et al (2016) CHAMPION-1: a phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood 127(26):3360–3368
Wang M, Martin T, Bensinger W, Alsina M, Siegel DS, Kavalerchik E et al (2013) Phase 2 dose-expansion study (PX-171-006) of carfilzomib, lenalidomide, and low-dose dexamethasone in relapsed or progressive multiple myeloma. Blood 122(18):3122–3128
Fiala MA, Keller J, Sekhar J, Ceriotti C, Abboud CN, DiPersio JF et al (2016) A phase II study of carfilzomib, pegylated liposomal doxorubicin, and dexamethasone for relapsed or refractory multiple myeloma. Blood 128:3329
Stewart AK, Rajkumar SV, Dimopoulos MA, Masszi T, Špička I, Oriol A et al (2015) Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med 372(2):142–152
Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R et al (2016) Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 17(1):27–38
Kumar SK, Berdeja JG, Niesvizky R, Lonial S, Laubach JP, Hamadani M et al (2014) Safety and tolerability of ixazomib, an oral proteasome inhibitor, in combination with lenalidomide and dexamethasone in patients with previously untreated multiple myeloma: an open-label phase 1/2 study. Lancet Oncol 15(13):1503–1512
Ludwig H, Gunsilius E, Fridrik M, Greil R, Petzer A, Kühr T et al (2016) Ixazomib, thalidomide and dexamethasone (IxaThalDex) in relapsed/refractory multiple myeloma (RRMM): an interim analysis of a phase II trial. Blood 128:3335
Kumar S, Grzasko N, Delimpasi S, Jędrzejczak WW, Grosicki S, Kyrtsonis M-C et al (2016) Phase 2 study of the all-oral combination of ixazomib plus cyclophosphamide and low-dose dexamethasone (ICd) in patients (Pts) with relapsed/refractory multiple myeloma (RRMM). Blood 128:3327
Kumar SK, LaPlant BR, Reeder CB, Roy V, Halvorson AE, Buadi F et al (2016) Randomized phase 2 trial of ixazomib and dexamethasone in relapsed multiple myeloma not refractory to bortezomib. Blood 128:2415–2422
Moreau P, Masszi T, Grzasko N, Bahlis NJ, Hansson M, Pour L et al (2016) Oral ixazomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 374(17):1621–1634
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Appendix
Appendix
Subgroup analysis of response rates derived from PIs + IMiDs + DEX vs. PIs + alkylating agent + DEX for relapsed/refractory multiple myeloma. (5.1.1, 5.1.2) Overall response rate (ORR) from PIs + IMiDs + DEX and PIs + alkylating agent + DEX. (5.2.1, 5.2.2) Very good partial response rate or better (≥ VGPR) from PIs + IMiDs + DEX and PIs + alkylating agent + DEX. (5.3.1, 5.3.2) Complete response or better rate (≥ CR) from PIs + IMiDs + DEX and PIs + alkylating agent + DEX. (5.4.1, 5.4.2) Clinical benefit rate (CBR) from PIs + IMiDs + DEX and PIs + alkylating agent + DEX. CI, 95% confidence interval; Random, random-effects model
Meta-analysis of carfilzomib-containing regimens vs. standard chemotherapy for relapsed/refractory multiple myeloma in terms of response outcomes. (6.1.1) Overall response rate (ORR) analysis. (6.1.2) Very good partial response or better rate (≥ VGPR) analysis. (6.1.3) Complete response or better rate (≥ CR) analysis. (6.1.3) Clinical benefit rate (CBR) analysis. CI, 95% confidence interval; Random, random-effects model
Rights and permissions
About this article
Cite this article
Xu, W., Sun, X., Wang, B. et al. Pooled analysis of the reports of carfilzomib/ixazomib combinations for relapsed/refractory multiple myeloma. Ann Hematol 97, 299–307 (2018). https://doi.org/10.1007/s00277-017-3173-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3173-9