Abstract
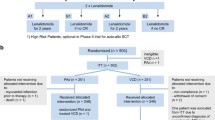
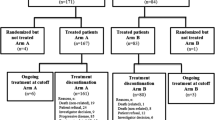
This phase III, open-label, randomized, controlled study aimed to evaluate the benefit of adding continuous low-dose oral cyclophosphamide to bortezomib-dexamethasone in patients with primary relapsed/refractory multiple myeloma. Patients were randomized 1:1 to receive up to eight 3-week cycles of bortezomib (1.3 mg/m2) and dexamethasone (20 mg; VD; n = 48) or bortezomib-dexamethasone plus oral cyclophosphamide (50 mg; VCD; n = 48). Median time to progression (primary endpoint) was slightly longer in the VD versus VCD group (12.6 vs 9.9 months, P = 0.192), and the hazard ratio for disease progression was in favor of VD (hazard ratio = 0.71, 95% confidence interval = 0.43–1.19, P = 0.196). The overall response rate was 74% with VD and 70% with VCD. Most adverse events were similar in frequency between arms; however, grade ≥ 3 peripheral neuropathy was more frequent in the VCD versus VD arm (15 vs 4%). Infection rate was higher in the VCD arm (64 vs 52%); however, grade ≥3 infection rates were comparable (19 vs 17%). Further trials are needed to determine whether addition of cyclophosphamide to VD at a different dose/schedule confers clinical benefit. This study was terminated prematurely, with insufficient sample size to adequately compare the arms; the results should, therefore, be considered descriptive. This trial is registered: EudraCT Number 2008-003213-27; ClinicalTrials.gov NCT00813150.


Similar content being viewed by others
References
Baz RC, Martin TG 3rd, Lin HY, Zhao X, Shain KH, Cho HJ, Wolf JL, Mahindra A, Chari A, Sullivan DM, Nardelli LA, Lau K, Alsina M, Jagannath S (2016) Randomized multicenter phase 2 study of pomalidomide, cyclophosphamide, and dexamethasone in relapsed refractory myeloma. Blood 127:2561–2568
Ciolli S, Leoni F, Gigli F, Rigacci L, Bosi A (2006) Low dose Velcade, thalidomide and dexamethasone (LD-VTD): an effective regimen for relapsed and refractory multiple myeloma patients. Leuk Lymphoma 47:171–173
Davies FE, Wu P, Jenner M, Srikanth M, Saso R, Morgan GJ (2007) The combination of cyclophosphamide, velcade and dexamethasone induces high response rates with comparable toxicity to velcade alone and velcade plus dexamethasone. Haematologica 92:1149–1150
Dimopoulos MA, Orlowski RZ, Facon T, Sonneveld P, Anderson KC, Beksac M, Benboubker L, Roddie H, Potamianou A, Couturier C, Feng H, Ataman O, van de Velde H, Richardson PG (2013a) Retrospective matched-pair analysis of the efficacy and safety of Bortezomib plus dexamethasone versus Bortezomib monotherapy in patients (pts) with relapsed multiple myeloma (MM). Blood (ASH Annual Meeting Abstracts) 121:3177
Dimopoulos MA, Beksac M, Benboubker L, Roddie H, Allietta N, Broer E, Couturier C, Mazier MA, Angermund R, Facon T (2013b) Phase II study of bortezomib-dexamethasone alone or with added cyclophosphamide or lenalidomide for sub-optimal response as second-line treatment for patients with multiple myeloma. Haematologica 98:1264–1272
Durie BG, Harousseau JL, Miguel JS, Blade J, Barlogie B, Anderson K, Gertz M, Dimopoulos M, Westin J, Sonneveld P, Ludwig H, Gahrton G, Beksac M, Crowley J, Belch A, Boccadaro M, Cavo M, Turesson I, Joshua D, Vesole D, Kyle R, Alexanian R, Tricot G, Attal M, Merlini G, Powles R, Richardson P, Shimizu K, Tosi P, Morgan G, Rajkumar SV (2006) International uniform response criteria for multiple myeloma. Leukemia 20:1467–1473
Einsele H, Liebisch P, Langer C, Kropff M, Wandt H, Jung W, Kroger N, Engelhardt M, Ostermann H, Mugge L-O, Wolf H-H, Hart C, Metzner B, Kaufmann M (2009) Velcade, intravenous cyclophosphamide and dexamethasone (VCD) induction for previously untreated multiple myeloma (German DSMM XIa trial). Blood (ASH Annual Meeting Abstracts) 114:131
Garderet L, Iacobelli S, Moreau P, Dib M, Lafon I, Niederwieser D, Masszi T, Fontan J, Michallet M, Gratwohl A, Milone G, Doyen C, Pegourie B, Hajek R, Casassus P, Kolb B, Chaleteix C, Hertenstein B, Onida F, Ludwig H, Ketterer N, Koenecke C, van Os M, Mohty M, Cakana A, Gorin NC, de Witte T, Harousseau JL, Morris C, Gahrton G (2012) Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 randomized phase III trial from the chronic leukemia working Party of the European Group for blood and marrow transplantation. J Clin Oncol 30:2475–2482
Gozzetti A, Candi V, Papini G, Bocchia M (2014) Therapeutic advancements in multiple myeloma. Front Oncol 4:241
Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R, Limentani SA, Alsina M, Adams J, Kauffman M, Esseltine DL, Schenkein DP, Anderson KC (2004) A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol 127:165–172
Jakubowiak A, Offidani M, Pégourie B, De La Rubia J, Garderet L, Laribi K, Bosi A, Marasca R, Laubach J, Mohrbacher A, Carella AM, Singhal AK, Tsao LC, Lynch M, Bleickardt E, Jou YM, Robbins M, Palumbo A (2016) Randomized phase 2 study: elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 127:2833–2840
Kropff M, Bisping G, Schuck E, Liebisch P, Lang N, Hentrich M, Dechow T, Kroger N, Salwender H, Metzner B, Sezer O, Engelhardt M, Wolf HH, Einsele H, Volpert S, Heinecke A, Berdel WE, Kienast J (2007) Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol 138:330–337
Kumar S, Flinn I, Richardson PG, Hari P, Callander N, Noga SJ, Stewart AK, Turturro F, Rifkin R, Wolf J, Estevam J, Mulligan G, Shi H, Webb IJ, Rajkumar SV (2012) Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood 119:4375–4382
Kumar SK, Therneau TM, Gertz MA, Lacy MQ, Dispenzieri A, Rajkumar SV, Fonseca R, Witzig TE, Lust JA, Larson DR, Kyle RA, Greipp PR (2004) Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc 79:867–874
Ludwig H, Kasparu H, Leitgeb C, Rauch E, Linkesch W, Zojer N, Greil R, Seebacher A, Pour L, Weissmann A, Adam Z (2014a) Bendamustine-bortezomib-dexamethasone is an active and well-tolerated regimen in patients with relapsed or refractory multiple myeloma. Blood 123:985–991
Ludwig H, Sonneveld P, Davies F, Blade J, Boccadoro M, Cavo M, Morgan G, de la Rubia J, Delforge M, Dimopoulos M, Einsele H, Facon T, Goldschmidt H, Moreau P, Nahi H, Plesner T, San-Miguel J, Hajek R, Sondergeld P, Palumbo A (2014b) European perspective on multiple myeloma treatment strategies in 2014. Oncologist 19:829–844
Ludwig H, Viterbo L, Greil R, Masszi T, Spicka I, Shpilberg O, Hajek R, Dmoszynska A, Paiva B, Vidriales MB, Esteves G, Stoppa AM, Robinson D Jr, Ricci D, Cakana A, Enny C, Feng H, van de Velde H, Harousseau JL (2013) Randomized phase II study of bortezomib, thalidomide, and dexamethasone with or without cyclophosphamide as induction therapy in previously untreated multiple myeloma. J Clin Oncol 31:247–255
Mai EK, Bertsch U, Durig J, Kunz C, Haenel M, Blau IW, Munder M, Jauch A, Schurich B, Hielscher T, Merz M, Huegle-Doerr B, Seckinger A, Hose D, Hillengass J, Raab MS, Neben K, Lindemann HW, Zeis M, Gerecke C, Schmidt-Wolf IG, Weisel K, Scheid C, Salwender H, Goldschmidt H (2015) Phase III trial of bortezomib, cyclophosphamide and dexamethasone (VCD) versus bortezomib, doxorubicin and dexamethasone (PAd) in newly diagnosed myeloma. Leukemia 29:1721–1729
Mellqvist UH, Lenhoff S, Johnsen HE, Hjorth M, Holmberg E, Juliusson G, Tangen JM, Westin J (2008) Cyclophosphamide plus dexamethasone is an efficient initial treatment before high-dose melphalan and autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of a randomized comparison with vincristine, doxorubicin, and dexamethasone. Cancer 112:129–135
Mikhael JR, Belch AR, Prince HM, Lucio MN, Maiolino A, Corso A, Petrucci MT, Musto P, Komarnicki M, Stewart AK (2009) High response rate to bortezomib with or without dexamethasone in patients with relapsed or refractory multiple myeloma: results of a global phase 3b expanded access program. Br J Haematol 144:169–175
Moreau P (2012) The future of therapy for relapsed/refractory multiple myeloma: emerging agents and novel treatment strategies. Semin Hematol 49(Suppl 1):S33–S46
Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine DL, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau JL (2011) Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. Lancet Oncol 12:431–440
Moreau P, Richardson PG, Cavo M, Orlowski RZ, San Miguel JF, Palumbo A, Harousseau JL (2012) Proteasome inhibitors in multiple myeloma: 10 years later. Blood 120:947–959
Moreau P, San MJ, Ludwig H, Schouten H, Mohty M, Dimopoulos M, Dreyling M (2013) Multiple myeloma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 24(Suppl 6):vi133–vi137
Niesvizky R, Flinn IW, Rifkin R, Gabrail N, Charu V, Clowney B, Essel J, Gaffar Y, Warr T, Neuwirth R, Zhu Y, Elliot J, Esseltine DL, Niculescu L, Reeves J (2015) Community-based phase IIIB trial of three UPFRONT Bortezomib-based myeloma regimens. J Clin Oncol 33:3921–3929
Offidani M, Corvatta L, Maracci L, Liberati AM, Ballanti S, Attolico I, Caraffa P, Alesiani F, Caravita di TT, Gentili S, Tosi P, Brunori M, Derudas D, Ledda A, Gozzetti A, Cellini C, Malerba L, Mele A, Andriani A, Galimberti S, Mondello P, Pulini S, Coppetelli U, Fraticelli P, Olivieri A, Leoni P (2013) Efficacy and tolerability of bendamustine, bortezomib and dexamethasone in patients with relapsed-refractory multiple myeloma: a phase II study. Blood Cancer J 3:e162
Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, Spicka I, Hungria V, Munder M, Mateos MV, Mark TM, Qi M, Schecter J, Amin H, Qin X, Deraedt W, Ahmadi T, Spencer A, Sonneveld P, CASTOR Investigators (2016) Daratumumab, Bortezomib, and dexamethasone for multiple myeloma. N Engl J Med 375:754–766
Pineda-Roman M, Zangari M, van Rhee F, Anaissie E, Szymonifka J, Hoering A, Petty N, Crowley J, Shaughnessy J, Epstein J, Barlogie B (2008) VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia 22:1419–1427
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Hentz J, Noble B, Pirooz NA, Spong JE, Piza JG, Zepeda VH, Mikhael JR, Leis JF, Bergsagel PL, Fonseca R, Stewart AK (2009) Cyclophosphamide, bortezomib and dexamethasone induction for newly diagnosed multiple myeloma: high response rates in a phase II clinical trial. Leukemia 23:1337–1341
Reeder CB, Reece DE, Kukreti V, Chen C, Trudel S, Laumann K, Hentz J, Pirooz NA, Piza JG, Tiedemann R, Mikhael JR, Bergsagel PL, Leis JF, Fonseca R, Stewart AK (2010) Once- versus twice-weekly bortezomib induction therapy with CyBorD in newly diagnosed multiple myeloma. Blood 115:3416–3417
Richardson PG, Hideshima T, Mitsiades C, Anderson KC (2007) The emerging role of novel therapies for the treatment of relapsed myeloma. J Natl Compr Cancer Netw 5:149–162
Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C, Mukhopadhyay S, Ondovik MS, Khan M, Paley CS, Lonial S (2013) PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma. Blood 122:2331–2337
Richardson PG, Xie W, Jagannath S, Jakubowiak A, Lonial S, Raje NS, Alsina M, Ghobrial IM, Schlossman RL, Munshi NC, Mazumder A, Vesole DH, Kaufman JL, Colson K, McKenney M, Lunde LE, Feather J, Maglio ME, Warren D, Francis D, Hideshima T, Knight R, Esseltine DL, Mitsiades CS, Weller E, Anderson KC (2014) A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood 123:1461–1469
Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC (2003) A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med 348:2609–2617
San-Miguel JF, Hungria VT, Yoon SS, Beksac M, Dimopoulos MA, Elghandour A, Jedrzejczak WW, Günther A, Nakorn TN, Siritanaratkul N, Corradini P, Chuncharunee S, Lee JJ, Schlossman RL, Shelekhova T, Yong K, Tan D, Numbenjapon T, Cavenagh JD, Hou J, LeBlanc R, Nahi H, Qiu L, Salwender H, Pulini S, Moreau P, Warzocha K, White D, Bladé J, Chen W, de la Rubia J, Gimsing P, Lonial S, Kaufman JL, Ocio EM, Veskovski L, Sohn SK, Wang MC, Lee JH, Einsele H, Sopala M, Corrado C, Bengoudifa BR, Binlich F, Richardson PG (2014) Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomised, double-blind phase 3 trial. Lancet Oncol 15:1195–1206
Takamatsu Y, Sunami K, Muta T, Morimoto H, Miyamoto T, Higuchi M, Uozumi K, Hata H, Tamura K (2013) Bortezomib, doxorubicin and intermediate-dose dexamethasone (iPAD) therapy for relapsed or refractory multiple myeloma: a multicenter phase 2 study. Int J Hematol 98:179–185
Uttervall K, Admasie J, Alici E, Lund J, Liwing J, Aschan J, Barendse M, Deneberg S, Mellqvist UH, Carlson K, Nahi H (2013) A combination regimen of bortezomib, cyclophosphamide and betamethasone gives quicker, better and more durable response than VAD/CyBet regimens: results from a Swedish retrospective analysis. Acta Haematol 130:7–15
Vincent RS (2014) Multiple myeloma: 2014 update on diagnosis, risk-stratification, and management. Am J Hematol 89:999–1009
Zhang Y, Liu H, Chen X, Bai Q, Liang R, Shi B, Liu L, Tian D, Liu M (2014) Modified bortezomib, adriamycin and dexamethasone (PAD) regimen in advanced multiple myeloma. Pathol Oncol Res 20:987–995
Acknowledgements
The authors would like to acknowledge the contribution of Astrid Kruse as study coordinator for the majority of the patients. The authors would also like to acknowledge the writing assistance of Helen Johns of FireKite, an Ashfield company, part of UDG Healthcare plc during the development of this publication, which was funded by Millennium Pharmaceuticals Inc., a wholly owned subsidiary of Takeda Pharmaceutical Company Limited, and Janssen Global Services, LLC. This study was supported by research funding from Janssen Research & Development and Millennium Pharmaceuticals, Inc.
Authorship contribution statements
MK was the principal investigator and takes primary responsibility for the paper; MK, GB, RS, LM, RW, WK, TD, HF, GK, SK, and WEB collected data and/or recruited patients; MK and WEB designed the study. MV co-ordinated preparation and analysis of the dataset; MK and WEB interpreted the data. MK, WEB, and MV prepared the manuscript. All authors reviewed and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was supported by research funding from Janssen Research & Development and Millennium Pharmaceuticals, Inc.
Conflict of interest
MK received honoraria, consultancy fees, and speaker’s bureau/advisory committee from Janssen, Celgene, and Mundipharma. WK received honoraria, consultancy fees, and speaker’s bureau/advisory committee from Janssen, Celgene, Mundipharma, and Roche. MV is an employee of Janssen; WEB received an honorarium from Janssen-Cilag. All other authors report no conflicts of interest.
Research involving human participants
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This article does not contain any studies with animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kropff, M., Vogel, M., Bisping, G. et al. Bortezomib and low-dose dexamethasone with or without continuous low-dose oral cyclophosphamide for primary refractory or relapsed multiple myeloma: a randomized phase III study. Ann Hematol 96, 1857–1866 (2017). https://doi.org/10.1007/s00277-017-3065-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-017-3065-z