Abstract
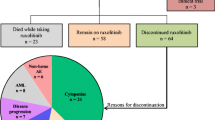
Ruxolitinib, a JAK1 and JAK2 inhibitor, has been tested and approved for the treatment of primary and secondary myelofibrosis (MF). Aim of our study is to report safety and efficacy of ruxolitinib in 98 patients affected by MF treated outside clinical trials and collected and treated consecutively by the Lazio Cooperative Group for Ph negative myeloproliferative diseases.There were 45 males and 53 females; median age was 61.8 years (range 35.3–88). Forty-five patients were diagnosed as primary MF and 53 as secondary MF. Seventy-seven patients (78.5%) experienced constitutional symptoms at baseline, and out of 94 patients tested, 66 (70%) were JAK2V617F mutated. Overall, 40 patients received hydroxyurea as firstline treatment, 30 patients received other chemotherapeutic approaches, whereas 28 were treated with ruxolitinib frontline. Median time from diagnosis to start of ruxolitinib in the whole cohort was 34.6 months. Fifty-eight patients (59%) required a dose reduction during the first 3 months due to hematological toxicity in the majority of cases. At 48 weeks, 52% of patients obtained a clinical benefit: of them 7 patients (7%) had a CR, 10 (10%) a PR, 6 patients (6%) a CI, and 28 patients (28.5%) a spleen response. Overall, 66% of patients had disappearance of baseline symptoms burden. After 1 year, of 72 evaluable patients, 52% achieved and maintained a clinical benefit. Adverse events of special interest at any grade included anemia (39.7%), thrombocytopenia (25.5%), infections (16.3%, of which 10 were bronchopneumonia), fluid retention (3%), diarrhea (2%) and abdominal pain (2%). After a median follow-up of 16 months from start of ruxolitinib, median daily dose decreased to 10 mg BID and 21 patients (21%) discontinued the drug. The results of this retrospective multicentric analysis confirmed the efficacy of ruxolitinib outside clinical trials with more than half of treated patients achieving and maintaining a clinical benefit and most of them reporting relief from symptoms.


Similar content being viewed by others
References
Passamonti F, Maffioli M, Caramazza D (2012) New generation small-moleculae inhibitors in myeloproliferative neoplasms. Curr Opin Hematol 19:117–123
Verstovsek S, Mesa RA, Gotlib J et al (2012) A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med 366:799–807
Cervantes F, Vannucchi AM, Kiladjian JJ et al (2013) Three-year efficacy, safety, and survival findings from COMFORT-II, a phase 3 study comparing ruxolitinib with best available therapy for myelofibrosis. Blood 122:4047–4053
Verstovsek S, Mesa RA, Gotlib J et al (2015) Efficacy and safety and survival with ruxolitinib in patients with myelofibrosis: results of a median 3-year follow-up of COMFORT-I. Haematologica 100:479–488
Harrison CN, Vannucchi AM, Kiladjian JJ et al (2016) Long-term findings from COMFORT-II, a phase 3 study of ruxolitinib vs best available therapy for myelofibrosis. Leukemia 30:1701–1708
Tavares R, Palumbo G, Le Coutre P et al (2015) Safety and efficacy of ruxolitinib in an 1869-patient cohort of JUMP: an open-label multicentre, single-arm, expanded access study in patients with myelofibrosis. Blood 126 abstract 2799
Vardiman JW, Thiele J, Arber DA et al (2009) The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 114:937–951
Tefferi A, Cervantes F, Mesa R et al (2013) Revised response criteria for myelofibrosis: international working group-myeloproliferative neoplasms research and treatment (IWG-MRT) and European LeukemiaNet (ELN) consensus report. Blood 122:1395–1398
Ellis MH, Lavi N, Mishchenko E, et al. (2015). Ruxolitinib treatment for myelofibrosis: efficacy and tolerability in routine practice. Leuk Res; Epub ahead of print Aug 12. pii: S0145–2126(15)30356–8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
M.B. received honoraria for specking by Novartis, Bristol Myers Squibb, Pfizer, and Ariad. All the other authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Breccia, M., Andriani, A., Montanaro, M. et al. Ruxolitinib in clinical practice for primary and secondary myelofibrosis: an analysis of safety and efficacy of Gruppo Laziale of Ph-negative MPN. Ann Hematol 96, 387–391 (2017). https://doi.org/10.1007/s00277-016-2884-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-016-2884-7