Abstract
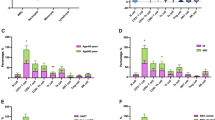
Hodgkin lymphoma (HL) is traditionally diagnosed by the presence of neoplastic Hodgkin and Reed-Sternberg (HRS) cells found in minority within a typical inflammatory microenvironment. It is now recognized that the majority of these T CD4 cells are T regulatory (Treg) and play an important immunosuppressive role and contribute to tumour persistence. Flow cytometric immunophenotyping of lymphocytes was performed on lymph node samples over a 12-year period (2000-2012) to identify the Hodgkin-specific subset and potential biomarkers related to Treg cells. CD3, CD19 and T CD4+CD26−CD38+ subsets were measured in the lymphocytic infiltrate of 108 consecutive lymph node samples concurrently diagnosed histologically as HL and in 43 cases of benign reactive lymphoid hyperplasia (BRLH). HL, compared to BRLH, shows statistically significant differences within the reactive microenvironmental population: decreased CD19+ cells (23 % vs 39 %; p < 0.001), increased CD3+ (74 % vs 58 %; p < 0.001) and CD4+CD26−CD38+ cells (38 % vs 11.5 %; p < 0.001). By using the co-expressed markers CD38 and CD26 for logistic analysis, the obtained receiver operating characteristic (ROC) curves confirm that the CD4+CD26−CD38+ subset is strongly expressed in HL (ROC AUC = 0,8639). Flow cytometric detection of CD4+CD26−CD38+ cells seems able to identify the cellular microenvironmental pattern in HL and to distinguish it from BRLH. Although there is extensive experience in flow cytometric analysis of non-HL, it is not routinely applied in cases of HL and our findings suggest that it may be useful in quickly and easily characterizing its cellular para-neoplastic inflammatory background.






Similar content being viewed by others
References
Gruss HJ, Pinto A, Duyster J, Poppema S, Herrmann F (1997) Hodgkin’s disease: a tumor with disturbed immunological pathways. Immunol Today 18:156–163
Swerdlow S, Campo E, Lee Harris N, Jaffe ES, Pileri SA, Stein H, Thiele J, Vardiman JW (2008) Classification of tumours of haematopoietic and lymphoid tissues, 4th edn. IARC, Lyon
Re D, Küppers R, Diehl V (2005) Molecular pathogenesis of Hodgkin’s lymphoma. J Clin Oncol 23:6379–6386
Chen-Chun S, Hsiu-Hui C, Chia-Che C, Jui-Chieh C, Su-Ming H (2004) CD30 is involved in inhibition of T-cell proliferation by Hodgkin’s Reed-Sternberg Cells. Cancer Res 64:2148–2152
Seegmiller AC, Karandikar NJ, Kroft SH, McKenna RW, Xu Y (2009) Overexpression of CD7 in classical Hodgkin lymphoma-infiltrating T lymphocytes. Cytometry B Clin Cytom 76:169–174
Fromm JR, Thomas A, Wood BL (2010) Increased expression of T cell antigens on T cells in classical Hodgkin lymphoma. Cytometry B Clin Cytom 78:387–388
Niens M, Visser L, Nolte IM, van der Steege G, Diepstra A, Cordano P, Jarret RF, Meerman GJ, Poppema S, van den Berg A (2008) Serum chemokine levels in Hodgkin lymphoma patients: highly increate levels of CCL17 and CCL22. B J Hae 140:527–536
Tanijiri T, Shimizu T, Uehira K, Yokoi T, Amuro H, Sugimoto H, Torii Y, Tajima K, Ito T, Amakawa R, Fukuhara S (2007) Hodgkin’s Reed-Sternberg cell line (KM-H2) promotes a bidirectional differentiation of CD4+CD25+Foxp3+ T cells and CD4+ cytotoxic T lymphocytes from CD4+ naive T cells. J Leu Biol 82:576–584
Skinnider BF, Mak TW (2002) The role of cytokines in classical Hodgkin lymphoma. Blood 99:4283–4297
Maggio E, van der Berg A, Diepstra A, Kluiver J, Visser L, Poppema S (2002) Chemokines, cytokines and their receptors in Hodgkin’s lymphoma cell lines and tissues. Ann Oncol 13:52–56
Hudnall SD, Betancourt E, Barnhart E, Patel J (2008) Comparative flow immunophenotypic features of the inflammatory infiltrates of Hodgkin lymphoma and lymphoid hyperplasia. Cytometry B Clin Cytom 74(1):1–8
Bosler DS, Douglas-Nikitin VK, Harris VN, Smith MD (2008) Detection of T-regulatory cells has a potential role in the diagnosis of classical Hodgkin lymphoma. Cytometry B Clin Cytom 74:227–235
Aldinucci D, Gloghini A, Pinto A, De Filippi R, Carbone A (2010) The classical Hodgkin’s lymphoma microenvironment and its role in promoting tumor growth and immune escape. J Pathol 221:248–263
Yurchenko M, Sidorenko SP (2010) Hodgkin’s lymphoma: the role of cell surface receptors in regulation of tumor cell fate. Exp Oncol 32:214–223
Lamprect B, Kreher S, Anagnostopoulos I, Jöhrens K, Monteleone G, Jundt F, Stein H, Janz M, Dörken B, Mathas S (2008) Aberrant expression of the TH2 cytokine IL-21 in Hodgkin lymphoma cells regulates STAT3 signaling and attracts Treg cells via regulation of MIP-3alpha. Blood 112:3339–3347
Marshall NA, Christie LE, Munro LR, Culligan LR, Jonston PW, Barker RN, Vickers MA (2004) Immunosuppressive regulatory T cells are abundant in the reactive lymphocytes of Hodgkin lymphoma. Blood 103:1755–1762
O’Garra A, Vieira P (2004) Regulatory T cells and mechanisms of immune system control. Nat Med 10:801–805
Ng WF, Duggan PJ, Ponchel F, Matarese G, Lombardi G, Edwards AD, Isaacs JD, Lechler RI (2001) Human CD4(+) CD25(+) cells: a naturally occurring population of regulatory T cells. Blood 98:2736–2744
Fontenot JD, Gavin MA, Rudensky AY (2003) Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 4:330–336
Wei WZ, Morris GP, Kong YC (2004) Anti-tumor immunity and autoimmunity: a balancing act of regulatory T cells. Cancer Immunol Immunother 53:73–78
Danese S, Rutella S (2007) The Janus face of CD4+CD25+ regulatory T cells in cancer and autoimmunity. Curr Med Chem 14:649–666
Whiteside TL (2008) The tumor microenvironment and its role in promoting tumor growth. Oncogene 27:5904–5912
Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC (2007) Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Me 204:1257–1265
Mandapathil M, Hilldorfer B, Miroslaw J, Szczepznski J, Czystowska M, Szaijnik M, Ren J, Lang S, Jackson EK, Gorelik E, Whiteside TL (2010) Generation and accumulation of immunosuppressive adenosine by human CD4+CD25highFOXP3+ regulatory T cells. J Biol Chem 285:7176–7186
Salgado FJ, Pérez-Díaz A, Villanueva NM, Lamas O, Arias P, Nogueira M (2012) CD26: a negative selection marker for human Treg cells. Cytometry 81:843–855
Poppema S, Potters M, Visser L, van den Berg AM (1998) Immune escape mechanisms in Hodgkin’s disease. Ann Oncol 9:21–24
Ma Y, Visser L, Blokzijl T, Harms G, Atayar C, Poppema S, van Den Berg A (2008) The CD4+CD26- T-cell population in classical Hodgkin’s lymphoma displays a distinctive regulatory T-cell profile. Lab Invest 88:482–490
Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G, van der Wal T, Luinge M, Alvarez-Llamas G, Vos H, Poppema S, Vonk R, van den Berg A (2008) Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood 111:2339–2346
Law JP, Hirschkorn DF, Owen RE, Biswas HH, Norris PJ, Lanteri MC (2009) The importance of Foxp3 antibody and fixation/permeabilization in identifying CD4+CD25+Foxp3+ regulatory T cells. Cytometry 75:1040–1050
Burger JA, Ghia P, Rosenwald A, Caligaris-Cappio F (2009) The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 114:3367–3375
Mantovani A, Alleva P, Sica P, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Steidl C, Connors JM, Gascoyne RD (2011) Molecular pathogenesis of Hodgkin’s lymphoma: increasing evidence of the importance of the microenvironment. J Cli Oncol 29:1812–1826
Beyer M, Schultze JL (2006) Regulatory T cells in cancer. Blood 108:804–811
Schreck S, Friebel D, Buettner M, Distel L, Grabenbauer G, Young LS, Niedobitek G (2009) Prognostic impact of tumour-infiltrating Th2 and regulatory T cells in classical Hodgkin lymphoma. Haemat Oncol 27:31–39
Sakaguchi S (2005) Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nature Immunol 6:345–352
Pillai V, Karandikar NJ (2008) Attack on the clones? Human FOXP3 detection by PCH101, 236A/E7, 206D, and 259D reveals 259D as the outlier with lower sensitivity. Blood 111:463–464
Ohkura N, Kitagawa Y, Sakaguchi S (2013) Development and maintenance of regulatory T cells. Immunity 38:414–423
Whiteside TL, Mandapathil M, Schuler P (2011) The role of the adenosinergic pathway in immunosuppression mediated by human regulatory T cells (Treg). Curr Med Chem 18:5217–5123
Sauer AV, Brigida I, Carriglio N, Aiuti A (2012) Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol 3:1–19
Sauer AV, Brigida I, Carriglio N, Hernandez RJ, Scaramuzza S, Clavenna D, Sanvito F, Poliani PL, Gagliani N, Carlucci F, Tabucchi A, Roncarolo MG, Traggiai E, Villa A, Aiuti A (2012) Alterations in the adenosine metabolism and CD39/CD73 adenosinergic machinery cause loss of Treg cell function and autoimmunity in ADA-deficient SCID. Blood 119:1428–1439
Mandapathil M, Szczepanski M, Harasymczuk M, Ren J, Cheng D, Jackson EK, Gorelik E, Johnson J, Lang S, Whiteside TL (2012) CD26 expression and adenosine deaminase activity in regulatory T cells (Treg) and CD4(+) T effector cells in patients with head and neck squamous cell carcinoma. Oncoimmunology 1:659–669
Mattern T, Scolz W, Feller AC (1991) Expression of CD26 (dipeptidyl peptidase IV) on resting and activation human T-lymphocytes. Scand J Immunol 33:737–748
Martinez A, Aymerich M, Castillo M, Colomer D, Bellosillo B, Campo E, Villamor N (2003) Routine use of immunophenotype by flow cytometry in tissues with suspected hematological malignancies. Cytometry Part B Clin Cytom 56B:8–15
Ravoet C, Demartin S, Gerard R, Dehon M, Peny MO, Petit B, Delannoy A, Husson B (2004) Contribution of flow cytometry to the diagnosis of malignant and non malignant conditions in lymph node biopsies. Leuk Lymphoma 45:1587–1593
Fromm JR, Thomas A, Wood BL (2009) Flow cytometry can diagnose classical Hodgkin lymphoma in lymph nodes with high sensitivity and specificity. Am J Clin Pathol 131:322–332
Acknowledgments
This work was in part supported by the “Avis per il progresso ematologico” (APE) and AIL Treviso. LC is a fellow of AIL Treviso and VG is a fellow of the “Borsa di studio ricerca sul cancro 2012 dedicata a don Umberto Miglioranza”, Castelfranco Veneto. We thank Gabrielle Talarico Clarke for the revision of this manuscript.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Di Gaetano, R., Gasparetto, V., Padoan, A. et al. Flow cytometry CD4+CD26−CD38+ lymphocyte subset in the microenvironment of Hodgkin lymphoma-affected lymph nodes. Ann Hematol 93, 1319–1326 (2014). https://doi.org/10.1007/s00277-014-2044-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-014-2044-x