Abstract
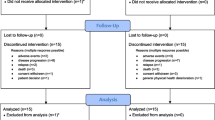
Previous studies have shown that clofarabine plus low-dose cytarabine (LDAC) could induce roughly 60 % of complete remissions (CR) with acceptable toxicity and induction mortality in elderly acute myeloid leukemia (AML) patients not suitable for intensive chemotherapy. The Programa Español de Tratamientos en Hematología group conducted a trial for patients diagnosed with untreated AML aged 60 years and older, using the combination of clofarabine (20 mg/m2 × 5 days) plus low-dose cytarabine (20 mg/m2 × 14 days). The protocol was flexible regarding the use of antifungal and antibacterial prophylaxis, and outpatient induction therapy was allowed. Although the planned recruitment goal was 75 patients, only 11 patients were enrolled (median age, 74 years) after observing high toxicity and unacceptable mortality (46 and 73 % at 4 and 8 weeks, respectively). The response assessment showed three CR (27 %), three resistant diseases (27 %), and five induction deaths (46 %). Induction was administered in an outpatient modality in five patients, while antifungal and antibacterial prophylaxis was not given in seven and five patients, respectively. In our context, induction therapy with the combination of clofarabine (20 mg/m2) plus LDAC was associated with high toxicity and unacceptable mortality in elderly AML patients, leading to the early interruption of the trial. Tight patients’ clinical monitoring, follow-up, and intensive supportive care seem crucial to achieve at least acceptable clinical outcomes in elderly AML patients receiving clofarabine plus LDAC. This trial is registered at www.clinicaltrials.gov as no. NCT01193400.
Similar content being viewed by others
References
NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesTM). Acute Myeloid Leukemia. Version 2.2013. http://www.nccn.org/index.asp. Accessed 16 April 2013
Burnett AK, Russell NH, Kell J, Dennis M, Milligan D, Paolini S et al (2010) European development of clofarabine as treatment for older patients with acute myeloid leukemia considered unsuitable for intensive chemotherapy. J Clin Oncol 28(14):2389–2395
Kantarjian HM, Erba HP, Claxton D, Arellano M, Lyons RM, Kovascovics T et al (2010) Phase II study of clofarabine monotherapy in previously untreated older adults with acute myeloid leukemia and unfavorable prognostic factors. J Clin Oncol 28(4):549–555
Faderl S, Ravandi F, Huang X, Garcia-Manero G, Ferrajoli A, Estrov Z et al (2008) A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood 112(5):1638–1645
Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al (2008) WHO Classification of tumours of haematopoietic and lymphoid tissues. IARC, Lyon
Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH et al (2003) Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol 21:4642–4649
Common terminology Criteria for Adverse Events (CTCAE). Version 4.0. http://ctep.cancer.gov/reporting/ctc.html. Accessed 16 April 2013
Faderl S, Ravandi F, Huang X, Wang X, Jabbour E, Garcia-Manero G et al (2012) Clofarabine plus low-dose cytarabine followed by clofarabine plus low-dose cytarabine alternating with decitabine in acute myeloid leukemia frontline therapy for older patients. Cancer 118(18):4471–4477
Acknowledgments
The authors thank Carlos Pastorini, David Pellicer, and Shirley Weiss for data collection and management. This study was supported in part by research funding from Instituto de Salud Carlos III grant R012/0036/0014 de la RTICC (Red Temática de Investigación Cooperativa en Cáncer)
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Trial registration: clinicaltrials.gov identifier: NCT01193400
Rights and permissions
About this article
Cite this article
Martínez-Cuadrón, D., Montesinos, P., Oriol, A. et al. Phase II trial to assess the safety and efficacy of clofarabine in combination with low-dose cytarabine in elderly patients with acute myeloid leukemia. Ann Hematol 93, 43–46 (2014). https://doi.org/10.1007/s00277-013-1914-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-013-1914-y