Abstract
This review focuses on the prevention and treatment of anemia in women who have just given childbirth (postpartum anemia). The problem of anemia both prepartum and postpartum is far more prevalent in developing countries than in the Western societies. The conditions for mother and child in the postpartum, nursing, and lactation period should be as favorable as possible. Many young mothers have a troublesome life due to iron deficiency and iron deficiency anemia (IDA) causing a plethora of symptoms including fatigue, physical disability, cognitive problems, and psychiatric disorders. Routine screening for postpartum anemia should be considered as part of the national maternal health programs. Major causes of postpartum anemia are prepartum iron deficiency and IDA in combination with excessive blood losses at delivery. Postpartum anemia should be defined as a hemoglobin level of <110 g/l at 1 week postpartum and <120 g/l at 8 weeks postpartum. Bleeding exceeding normal blood losses of approximately 300 ml may lead to rapid depletion of body iron reserves and may, unless treated, elicit long-standing iron deficiency and IDA in the postpartum period. The prophylaxis of postpartum anemia should begin already in early pregnancy in order to ensure a good iron status prior to delivery. The most reliable way to obtain this goal is to give prophylactic oral ferrous iron supplements 30–50 mg daily from early pregnancy and take obstetric precautions in pregnancies at risk for complications. In the treatment of slight-to-moderate postpartum IDA, the first choice should be oral ferrous iron 100 to 200 mg daily; it is essential to analyze hemoglobin after approximately 2 weeks in order to check whether treatment works. In severe IDA, intravenous ferric iron in doses ranging from 800 to 1,500 mg should be considered as first choice. In a few women with severe anemia and blunted erythropoiesis due to infection and/or inflammation, additional recombinant human erythropoietin may be considered. Blood transfusion should be restricted to women who develop circulatory instability due to postpartum hemorrhage. National health authorities should establish guidelines to combat iron deficiency in pregnancy and postpartum in order to facilitate a prosperous future for both mothers and children in a continuing globalized world.



Similar content being viewed by others
References
Milman N (2011) Postpartum anemia I: definition, prevalence, causes and consequences. Ann Hematol 90:1247–1253
Milman N. Anemia—still a major health problem in many parts of the world! Ann Hematol 2011; 90:369–377. http://dx.doi.org/10.1007/s00277-010-1144-5
World Health Organization (1999) Reduction of maternal mortality. A joint WHO/UNFPA/UNICEF/World Bank statement. World Health Organization, Geneva
Potts M, Campbell M (2004) Three meetings and fewer funerals: misoprostol in postpartum hemorrhage. Lancet 364:1110–1111
Tsu VD, Shane B (2004) New and underutilized technologies to reduce maternal mortality: call to action from a Bellagio workshop. Int J Gynecol Obstet 85(Suppl 1):S83–S93
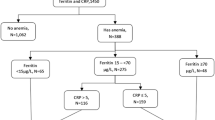
Bodnar LM, Siega-Riz AM, Miller WC, Cogswell ME, McDonald T (2002) Who should be screened for postpartum anemia? An evaluation of current recommendations. Am J Epidemiol 156:903–912
Bergmann RL, Richter R, Bergmann KE, Dudenhausen JW (2010) Prevalence and risk factors for early postpartum anemia. Eur J Obstet Gynecol Reprod Biol 150:126–131
Milman N, Kirchhoff M (1992) Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol 64:22–227
Milman N (2008) Prepartum anaemia: prevention and treatment. Ann Hematol 87:949–959
Oyelese Y, Ananth CV (2010) Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol 53:147–156
Danish Society for Obstetrics and Gynaecology http://www.dsog.dk
Milman N, Agger OA, Nielsen OJ (1991) Iron supplementation during pregnancy. Effect on iron status markers, serum erythropoietin and human placental lactogen. A placebo controlled study in 207 Danish women. Dan Med Bull 38:471–476
Åkesson A, Bjellerup P, Berglund M, Bremme K, Vahter M (2002) Soluble transferrin receptor: longitudinal assessment from pregnancy to postlactation. Obstet Gynecol 99:260–266
World Health Organization. Iron deficiency anemia. Assessment, prevention and control. 2001;WHO/NHD/01.3
Milman N, Bergholt T, Eriksen L, Byg K-E, Graudal N, Pedersen P, Hertz J (2005) iron prophylaxis during pregnancy—how much iron is needed? A randomised, controlled study of 20 to 80 mg ferrous iron daily to pregnant women. Acta Obstet Gynecol Scand 84:238–247
Milman N (2006) Iron and pregnancy—a delicate balance. Ann Hematol 85:559–565
Bothwell TH (2000) Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr 72:257S–264S
Siimes MA, Vuori E, Kuitunen P (1979) Breast milk iron—a declining concentration during the course of lactation. Acta Paed Scand 68:29–31
Pedersen AN, Fagt S, Groth MV, Christensen T, Biltoft-Jensen, Matthiessen J, et al. Dietary habits in Denmark 2003–2008. Main results: National Food Agency of Denmark. 2010
Commission of the European Communities. Nutrient and energy intakes for the European Community. Reports of the Scientific Committee for Food. Directorate General Industry 1993;31st series:180–181
National Collaborating Centre for Women's & Children's Health. NICE Clinical Guideline 62—Antenatal Care; March 2008
Social-og helsedirektoratet. Retningslinjer for svangerskapsomsorgen. www.shdir.no/publikasjoner Accessed December 2008
Recommendations CDC (1998) to prevent and control iron deficiency in the United States. Centers for Disease Control and Prevention. MMWR Recomm Rep 47:1–29
International Food information Council Foundation. Healthy eating during pregnancy. www.foodinsight.org Accessed January 2009
www.altomkost.dk Accessed January 2010
www.meraadet.dk Accessed March 2008
Swedish Society for Obstetrics and Gynecology. Maternal health, sexual and reproductive health (Mödrahälsovård, sexuell och reproduktiv hälsa) 2008;rapport no. 59:47–48
Trygg K, Lund-Larsen K, Sandstad B, Hoffman HJ, Jacobsen G, Bakketeig LS (1995) Do pregnant smokers eat differently from pregnant non-smokers? Paediatr Perinat Epidemiol 9:307–319
Nordic Council of Ministers. Nordic Nutrition Recommendations 2004. Copenhagen 2004
Milman N, Byg K-E, Bergholt T, Eriksen L, Hvas A-M (2006) Body iron and individual iron prophylaxis in pregnancy—should the iron dose be adjusted according to serum ferritin? Ann Hematol 85:567–573
Milman N (2006) Iron prophylaxis in pregnancy—general or individual and in which dose? Ann Hematol 85:821–828
Breymann C, Honegger C, Holzgreve W, Surbek D. Diagnostik und Therapie der Anämie in der Schwangerschaft und postpartal. Schweizerische Gesellschaft für Gynäkologie und Geburtshilfe. Expertenbrief 2007 no. 22
Beris P, Maniatis A, on behalf of the NATA working group on intravenous iron therapy. Guidelines on intravenous iron supplementation in surgery and obstetrics/gynecology. Transfusion Alternatives in Transfusion Medicine 2007;9 Suppl 1:29
ACOG Practice Bulletin (2006) clinical management guidelines for obstetrician/gynecologists number 76, 2006: postpartum hemorrhage. Obstet Gynecol 108:1039–1047
L Royal College of Obstetricians and Gynaecologist, Green top Guidelines, November 2009 http://www.rcog.org.uk/files/rcog-corp/Green-top52PostpartumHaemorrhage.pdf
Rajan PV, Wing DA (2010) Postpartum hemorrhage: evidence-based medical interventions for prevention and treatment. Clin Obstet Gynecol 53:165–181
Russell RT. WHO guidelines for the management of postpartum haemorrhage and retained placenta. World Health Organization WHO Press, Geneva, Switzerland, 2009, ISBN 978-92-4-159851-4
Dar S, Vardi IS, Holcberg G, Reuveni H, Yerushalmi R, Katz M, Sheiner E (2006) Do we need routine complete blood count following vaginal delivery? Int J Fertil Womens Med 51:270–273
Recommendation to prevent and control iron deficiency in the United States. MMWR Morb Mortal Wkly Rep 1998; 47(RR-3):13–25
Dodd JM, Dare MR, Middleton P. Treatment for women with postpartum iron deficiency anemia. Cochrane Database of Systematic Reviews 2004, Issue 4. Art. No.: CD004222. DOI: 10.1002/14651858.CD004222.pub2
Skikne B, Baynes RD (1994) Iron absorption. In: Brock JH, Halliday JW, Pippard MJ, Powell LW (eds) iron metabolism in health and disease. Saunders, London, pp 151–187
Milman N, Graudal N, Nielsen OJ, Agger AO (1997) Serum erythropoietin during normal pregnancy: relationship to hemoglobin and iron status markers and impact of iron supplementation in a longitudinal, placebo-controlled study on 118 women. Int J Hematol 66:159–168
Krafft A, Breymann C (2011) Iron sucrose with and without recombinant erythropoietin for the treatment of severe postpartum anemia: a prospective, randomized, open-label study. J Obstet Gynaecol Res 37:119–124
Nielsen JB, Ikkala E, Sölvell L, Björn-Rasmussen E, Ekenved G (1976) Absorption of iron from slow-release and rapidly-disintegrating tablets—a comparative study in normal subjects, blood donors and subjects with iron deficiency anemia. Scand J Haematol Suppl 28:89–97
Venofer®. Summary of Product Characteristics www.medicines.org.uk Accessed February 6th 2011
CosmoFer®. Summary of Product Characteristics www.medicines.org.uk Accessed February 6th 2011
Ferinject®. Summary of Product Characteristics www.medicines.org.uk Accessed February 6th 2011
Lyseng-Williamson KA, Keating GM (2009) Ferric carboxymaltose: a review of its use in iron-deficiency anemia. Drugs 69:739–756
Monofer®. Summary of Product Characteristics www.medicines.org.uk Accessed February 6th 2011
al-Momen AK, al-Meshari A, al-Nuaim L, Saddique A, Abotalib Z, Khashogji T et al (1996) Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur J Obstet Gynecol Reprod Biol 69:121–124
al-Ragip A, Unlubilgin E, Kandemir O, Yalvac S, Cakir L, Haberal A (2005) Intravenous versus oral iron for treatment of anemia in pregnancy: a randomized trial. Obstet Gynecol 106:1335–1340
Hallak M, Sharon A, Duikman R, Auslender R, Abramovici H (1997) Supplementation iron intravenously in pregnancy. A way to avoid blood transfusions. J Reprod Med 42:99–103
Breymann C, Visca E, Huch R, Huch A (2001) Efficacy and safety of intravenously administered iron sucrose with and without adjuvant recombinant human erythropoietin for the treatment of resistant iron-deficiency anemia during pregnancy. Am J Obstet Gynecol 184:662–667
Bashiri A, Burstein E, Sheiner E, Mazor M (2003) Anemia during pregnancy and treatment with intravenous iron: review of the literature. Eur J Obstet Gynecol Reprod Biol 110:2–7
Van Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A (2007) Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia. A randomised controlled trial. Obstet Gynecol 110:267–278
Breymann C, Richter C, Hüttner C, Huch R, Huch A (2000) Effectiveness of recombinant erythropoietin and iron sucrose vs. iron therapy only, in patients with postpartum anemia and blunted erythropoiesis. Eur J Clin Invest 30:154–161
Bhandal N, Russell R (2006) Intravenous versus oral iron therapy for postpartum anemia. BJOG 113:1248–1252
Giannoulis C, Daniilidis A, Tantanasis T, Dinas K, Tzafettas J (2009) Intravenous administration of iron sucrose for treating anemia in postpartum women. Hippokratia 13:38–40
Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R (2008) Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. Am J Obst Gynecol 199:435e1–435e7
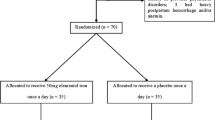
Westad S, Backe B, Salvesen KÅ, Nakling J, Økland I, Borthen I et al (2008) A 12-week randomised study comparing intravenous iron sucrose versus oral ferrous sulphate for treatment of postpartum anemia. Acta Obstet Gynecol Scand 87:916–923
Ganzoni AM (1972) Totalinfusion von Ferri-Kohlenhydrat-Komplexen (Total infusion of ferri-carbohydrate complexes). Blut 25:349–351
Swedish Dental and Pharmaceutical Benefits Agency http://www.tlv.se/in-english Accessed February 6th 2011
Kotto-Kome AC, Calhoun DA, Montenegro R, Sosa R, Maldonado L, Christensen RD (2004) Effect of administering recombinant erythropoietin to women with postpartum anemia: a meta-analysis. J Perinatol 24:11–15
Wågström E, Åkesson A, Van Rooijen M, Larson B, Bremme K (2007) Erythropoietin and intravenous iron therapy in postpartum anemia. Acta Obstet Gynecol Scand 86:957–962
Lebrecth A, Häberlin f, Eberhard J (1995) Anämie im Wochenbett; parenterale Eisensubstitution macht Erythropoietin-Therapie entberlich (Postpartum anaemia; intravenous iron supplementation renders therapy with rhEPO redundant). Geburtshilfe Frauenheilkd 55:167–170
Breymann c, Zimmermann R, Huch A (1996) Use of recombinant erythropoietin in combination with parenteral iron in the treatment of postpartum anemia. Eur J Clin Invest 26:123–130
Macrydimas G, Lolis G, Lialios G, Tsiara S, Georgiou I, Bourantas KL (1998) Recombinant human erythropoietin treatment of postpartum anemia. Preliminary results. Eur J Obstet Gynecol Reprod Biol 81:27–31
Gyamfi C, Berkowitz RL (2007) Management of pregnancy in a Jehova's Witness. Obs Gynecol Clin N Am 34:357–365
Bunn HF (2009) End run about Epo. New Engl J Med 361:1901–1903
Roberts CL, Ford JB, Thomson JF, Morris JM (2008) Population rates of hemorrhage and transfusions among obstetric patients in NSW: a short communication. Aust N Z J Obstet Gynaecol 48:481–484
Al-Zirqi I, Vangen S, Forsen L, Stray-Pedersen B (2008) Prevalence and risk factors of severe obstetric hemorrhage. BJOG 115:1265–1272
Rouse DJ, MacPherson C, Landon M, Varner MW, Leveno KJ, Moawad AH et al (2006) Blood transfusion and cesarean delivery. Obstet Gynecol 108:891–897
Fuller AJ, Bucklin BA (2010) Blood product replacement for postpartum hemorrhage. Clin Obstet Gynecol 53:196–208
Conflicts of interest
The author has given educational lectures for Abbott International, Pharmacosmos Ltd., and Vifor Pharma Ltd.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milman, N. Postpartum anemia II: prevention and treatment. Ann Hematol 91, 143–154 (2012). https://doi.org/10.1007/s00277-011-1381-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1381-2