Abstract
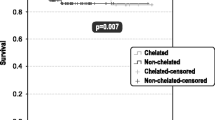
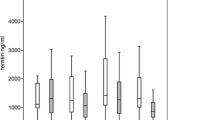
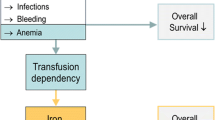
Transfusion dependency and iron overload are common among patients with myelodysplastic syndromes (MDS) treated with red blood cell (RBC) transfusions. Transfusion dependency is associated with leukemic progression and shorter survival. Guidelines recommend iron chelation therapy to manage iron overload, however little is known about the chelation patterns in daily clinical practice. The objective of this multicenter, retrospective, cross-sectional, observational study was to evaluate iron status and its management in transfusion-dependent MDS patients. A total of 193 patient records from 29 centers were eligible for inclusion. Median patient age was 76, and median age at diagnosis of MDS was 74. Patients had received an average of 13.4 ± 7.6 RBC units in the past 4 months; 44% had received more than 50 units since their MDS diagnosis. Medium serum ferritin was 1,550 μg/L. Ninety patients (46.6%) received iron chelation therapy with either deferoxamine (41%), deferasirox (36%), and deferoxamine followed by deferasirox (23%). There were no statistically significant differences between chelated and nonchelated patients in terms of International Prognostic Scoring System (IPSS), French-American-British (FAB), and/or World Health Organization (WHO) status, though chelated patients had received more RBC transfusions (p = 0.014). Iron chelation therapy may be underutilized in transfusion-dependent patients. Undertreatment can be reduced by complementing sound clinical judgment with the generally accepted guidelines of a serum ferritin level >1,000 μg/L and/or two or more RBC transfusions per month for the past year; considering patients on the basis of their IPSS, FAB, and/or WHO status; and individually tailored treatment regimens. Prospective randomized trials are necessary to establish causally the efficacy of iron chelation therapy in MDS.



Similar content being viewed by others
References
Kasner MT, Luger SM (2009) Update on the therapy for myelodysplastic syndrome. Am J Hematol 84:177–186
List AF (2006) Identifying best practices in the monitoring and treatment of iron overload in myelodysplastic syndrome. Hematol Oncol Clin North Am 20(Suppl 1):8–14
Malcovati L (2007) Impact of transfusion dependency and secondary iron overload on the survival of patients with myelodysplastic syndromes. Leuk Res 31(Suppl 3):S2–S6
Malcovati L, Della Porta MG, Pascutto C et al (2005) Prognostic factors and life expectancy in myelodysplastic syndromes classified according to the WHO criteria: a basis for clinical decision making. J Clin Oncol 23:7594–7603
Steensma DP, Bennett JM (2006) The myelodysplastic syndromes: diagnosis and treatment. May Clin Proc 81:104–130
Heaney ML, Golde DW (1999) Myelodysplasia. N Engl J Med 340:1649–1660
Bennett JM, Komrokji R, Kouides PA (2004) The myelodysplastic syndrome. In: Abeloff MD, Armitage JO, Niederhuber JE (eds) Clinical oncology, 3rd edn. Churchill Livingstone, New York, pp 2849–2881
Bennett JM, Catovsky D, Daniel MT et al (1982) Proposals for the classification of the myelodysplastic syndromes. Br J Haematol 51:189–199
Komrokji R, Bennett JM (2003) The myelodysplastic syndromes: classification and prognosis. Curr Hematol Rep 2:179–185
Germing U, Strupp C, Kuendgen A et al (2004) No increase in age-specific incidence of myelodysplastic syndromes. Haematologica 89:905–910
SEER Cancer Statistics Review 1975–2006. Available at: http://www.seer.cancer.gov/csr/1975_2006/results_merged/sect_30_mds.pdf. Accessed 15 April 2010
Greenberg P, Cox C, LeBeau MM et al (1997) International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2089–2088
Bennett JM (2008) For the MDS Foundation’s Working Group on Transfusional Iron Overload. Consensus statement on iron overload in myelodysplastic syndromes. Am J Hematol 83:858–861
Alessandrino EP, Amadori S, Barosi G et al (2002) Evidence- and consensus-based practice guidelines for the therapy of primary myelodysplastic syndromes. A statement from the Italian Society of Hematology. Haematologica 87:1286–1306
Malcovati L, Germing U, Kuendgen A et al (2007) Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol 35:3503–3510
Jabbour E, Kantarjian HM, Koller C, Taher A (2008) Red blood cell transfusions and iron overload in the treatment of patients with myelodysplastic syndromes. Cancer 112:1089–1095
Cazzola M, Malcovati L (2005) Myelodysplastic syndromes—coping with ineffective hematopoiesis. N Engl J Med 352:536–538
Suzuki T, Tomonaga M, Miyazaki Y et al (2008) Japanese epidemiological survey with consensus statement on Japanese guidelines for treatment of iron overload in bone marrow failure syndromes. Int J Hematol 88:30–35
Hoffbrand AV (2005) Deferiprone therapy for transfusional iron overload. Best Pract Res Clin Haematol 18:299–317
Porter JB (2001) Practical management of iron overload. Br J Haematol 115:239–252
Farquhar MJ, Bowen DT (2003) Oxidative stress and the myelodysplastic syndromes. Int J Hematol 77:342–350
Andrews NC (1999) Disorders of iron metabolism. N Engl J Med 341:1986–1995
Bowen D, Culligan D, Jowitt S et al (2003) Guidelines for the diagnosis and therapy of adult myelodysplastic syndromes. Br J Haematol 120:187–200
National Comprehensive Cancer Network (2010) NCCN clinical practice guidelines in oncology—v.2.2010. Myelodysplastic syndromes. Available at http://www.nccn.org/professionals/physician_gls/PDF/mds.pdf
Gattermann N, Porter J, Lopes L, Seymour J (2005) Consensus statement on iron overload in myelodysplastic syndromes. Hematol Oncol Clin North Am 19(Suppl 1):18–25
Santini V, Alessandrino PE, Angelucci E et al (2010) Clinical management of myelodysplastic syndromes: update of SIE, SIES, GITMO practice guidelines. Leuk Res 34:1576–1588
Gattermann N (2008) Overview of guidelines on iron chelation therapy in patients with myelodysplastic syndromes and transfusional iron overload. Int J Hematol 88:24–29
Pullarkat V (2009) Objectives of iron chelation therapy in myelodysplastic syndromes: more than meets the eye? Blood 114:5251–5255
Ceci A, Baiardi P, Felisi M et al (2002) The safety and effectiveness of deferiprone in a large-scale, 3-year study in Italian patients. Br J Haematol 118:330–336
Taher A, Cappellini MD (2009) Update on the use of deferasirox in the management of iron overload. Ther Clin Risk Manag 5:857–868
Cohen AR, Galanello R, Piga A et al (2003) Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood 102:1583–1587
Gattermann N, Finelli C, Della Porta M et al (2010) Deferasirox in iron-overloaded patients with transfusion-dependent myelodysplastic syndromes: results from the large 1-year EPIC study. Leuk Res 34:1143–1150
Greenberg PL, Koller CA, Cabantchik ZI et al (2010) Prospective assessment of effects of iron-overload parameters of deferasirox therapy in patients with myelodysplastic syndromes. Leuk Res 34:1560–1565
Remacha AF, Arrizabalaga B, Del Cañizo C et al (2010) Iron overload and chelation therapy in patients with low-risk myelodysplastic syndromes with transfusion requirements. Ann Hematol 89:147–154
Tefferi A, Stone RM (2009) Iron chelation therapy in myelodysplastic syndrome—cui bono? Leukemia 23:1373
Tefferi A, Vardiman JW (2009) Myelodysplastic syndromes. N Engl J Med 361:1872–1885
Tefferi A (2010) Myelodysplastic syndromes—many new drugs, little therapeutic progress. Mayo Clin Proc 85:1042–1045
Benson K, Hartz AJ (2000) A comparison of observational studies and randomized, controlled trials. N Engl J Med 342:1878–1886
Concato J, Shah N, Horwitz RI (2000) Randomized, controlled trials, observational studies, and the hierarchy of research designs. N Engl J Med 342:1887–1892
Jensen PD, Heickendorff L, Pederson B et al (1996) The effect of iron chelation on haemopoiesis in MDS patients with transfusional iron overload. Br J Haematol 94:288–299
Jensen PD, Jensen FT, Chistensen T et al (2003) Evaluation of myocardial iron by magnetic resonance imaging during iron chelation therapy with deferrioxamine: indication os close relation between myocardial iron content and chelatable iron pool. Blood 101:4632–4639
Olivieri NF, Brittenham GM (1997) Iron-chelating therapy and the treatment of thalassemia. Blood 89:739–761
Delea TE, Edelsberg J, Sofrygin O et al (2007) Consequences and costs of non compliance with iron chelation therapy in patients with transfusion-dependent thalassemia: a literature review. Transfusion 47:1919–1929
Gonzáles FA, Arrizabalaga B, Villegas A et al (2005) Study of deferoxamine in subcutaneous profusion treatment of iron overload in myelodysplastic syndromes. Med Clin Barc 124:645–647
Cappellini MD, Taher A (2009) Deferasirox (Exjade) for the treatment of iron overload. Acta Haematol 122:165–173
Cappellini MD, Cohen A, Piga A et al (2006) A phase three study of deferasirox (ICL670), a once-daily oral iron chelator, in patients with beta-thalassemia. Blood 107:3455–3462
Acknowledgments
This study was sponsored by Novartis Pharma. The authors thank the other investigators in this study, as well as their staff: Marc André, Charleroi; Yves Beguin, Liège; Dominique Boulet, Mons; Dimitri Breems, Antwerpen; Randal D’Dondt, Oostende ; Robrecht De Bock, Wilrijk ; Dries Deeren, Roeselare ; Anne Deweweire, Baudour ; André Efira, Brussel; Walter Feremans, Bruxelles; Augustin Ferrant, Woluwe; Kurt Geldhof, Ieper; Jan Lemmens, Wilrijk; Lucien Noens, Gent; Marjan Petrick, Gent; Pascal Pierre, Arlon; Ann Van de Velde, Edegem; Koen Van Eygen, Kortrijk; Steven Van Steenweghen, Liège; Wim Wynendaele, Bonheiden; all in Belgium. The authors also thank Erin Arizmendi for her editorial, proofreading, and administrative assistance.
Disclosures of conflicts of interest
All authors completed the ICMJE uniform disclosure form for potential conflicts of interest. W. Pluymers is an employee of Novartis Pharma. Abraham and K. MacDonald are employees of Matrix45. By company policy, they are prohibited from owning equity in client organizations (except through mutual funds or other independent collective investment instruments) or contracting independently with client organizations. Matrix45 provides similar services to other biopharmaceutical companies on a nonexclusivity basis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Prior dissemination
Poster presentation at the 2009 Congress of the European Haematological Association, Berlin, Germany, June 4–7, 2009. Concurrent abstract published as: Delforge M, Selleslag D, Triffet A, et al. The use of iron chelation therapy for transfusion-dependent myelodysplastic syndrome patients: a cross-sectional study in Belgium. Haematologica, 2009;94: abstract 0806
Michel Delforge, Dominik Selleslag, and Christophe Ravoet, on behalf of the Belgian Hematology Society.
Rights and permissions
About this article
Cite this article
Delforge, M., Selleslag, D., Triffet, A. et al. Iron status and treatment modalities in transfusion-dependent patients with myelodysplastic syndromes. Ann Hematol 90, 655–666 (2011). https://doi.org/10.1007/s00277-011-1164-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-011-1164-9