Abstract
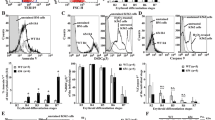
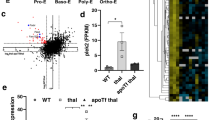
Erythropoiesis in β-thalassemia patients is ineffective, primarily because of death of the erythroid progenitor cells at the polychromatic normoblast stage. While it is known that autophagy plays a critical role during erythropoiesis by removing organelles from erythroid cells during terminal differentiation, its role in erythroid cells whose function is impaired remains to be explored. To investigate this, CD34+ erythroid progenitor cells from normal controls and β-thalassemia/Hb E patients were isolated from peripheral blood and cultured under conditions driving differentiation into an erythroid lineage, and levels of autophagy and apoptosis were analyzed both directly and after biochemical manipulation with l-asparagine. A significantly higher level of autophagy was seen in β-thalassemia/Hb E erythroblasts as compared to normal control erythroblasts during erythropoiesis. Interestingly, this activation was mediated in part by the presence of high levels of Ca2+ as modulation of Ca2+ levels significantly reduced the level of autophagy in these cells. Inhibition of autophagic flux in normal erythroblasts significantly increased apoptosis in normal erythroblasts, but not in thalassemic erythroblasts, although sensitivity to autophagic flux inhibition was restored by reduction of Ca2+ levels. These results suggest that high levels of autophagy in β-thalassemia/HbE erythroblasts may contribute to the increased levels of apoptosis that lead to ineffective erythropoiesis in β-thalassemia/Hb E erythroblasts.





Similar content being viewed by others
References
Mizushima N, Klionsky DJ (2007) Protein turnover via autophagy: implications for metabolism. Annu Rev Nutr 27:19–40. doi:10.1146/annurev.nutr.27.061406.093749
Mizushima N, Yoshimori T (2007) How to interpret lc3 immunoblotting. Autophagy 3(6):542–545
Klionsky DJ, Abeliovich H, Agostinis P, Agrawal DK, Aliev G, Askew DS et al (2008) Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy 4(2):151–175. 5338
Lerena C, Calligaris SD, Colombo MI (2008) Autophagy: for better or for worse, in good times or in bad times. Curr Mol Med 8(2):92–101
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451(7182):1069–1075. doi:10.1038/nature06639
Klionsky DJ, Emr SD (2000) Autophagy as a regulated pathway of cellular degradation. Science 290(5497):1717–1721
Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB (2008) Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ 15(1):171–182. doi:10.1038/sj.cdd.4402233
Zhang J, Ney PA (2009) Role of bnip3 and nix in cell death, autophagy, and mitophagy. Cell Death Differ 16(7):939–946. doi:10.1038/cdd.2009.16
Hoyer-Hansen M, Bastholm L, Szyniarowski P, Campanella M, Szabadkai G, Farkas T, Bianchi K, Fehrenbacher N, Elling F, Rizzuto R, Mathiasen IS, Jaattela M (2007) Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-beta, and bcl-2. Mol Cell 25(2):193–205. doi:10.1016/j.molcel.2006.12.009
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ros): implications for cancer progression and treatment. Antioxid Redox Signal 11(4):777–790. doi:10.1089/ARS.2008.2270
Scherz-Shouval R, Elazar Z (2007) Ros, mitochondria and the regulation of autophagy. Trends Cell Biol 17(9):422–427
Fimia GM, Piacentini M (2010) Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol Life Sci 67(10):1581–1588. doi:10.1007/s00018-010-0284-z
Gibbons R, Higgs DR, Olivieri NF, Wood WG (2001) The β thalassaemias. In: Weatherall DJ, Clegg JB (eds) The thalassaemia syndromes, 4th edn. Blackwell Science, Oxford, pp 287–356
Gibbons R, Higgs DR, Olivieri NF, Wood WG (2001) The β thalassaemias. In: Weatherall DJ, Clegg JB (eds) The thalassaemia syndromes 4th edn. Blackwell Science, Oxford, pp 287–356
Fucharoen S, Winichagoon P (2002) Thalassemia and abnormal hemoglobin. Int J Hematol 76(Suppl 2):83–89
Wannatung T, Lithanatudom P, Leecharoenkiat A, Svasti S, Fucharoen S, Smith DR (2009) Increased erythropoiesis of beta-thalassaemia/hb E proerythroblasts is mediated by high basal levels of erk1/2 activation. Br J Haematol 146(5):557–568
Lithanatudom P, Leecharoenkiat A, Wannatung T, Svasti S, Fucharoen S, Smith DR (2010) A mechanism of ineffective erythropoiesis in beta-thalassemia/hb E disease. Haematologica 95(5):716–723. doi:10.3324/haematol.2009.015701
Hoyer-Hansen M, Jaattela M (2007) Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ 14(9):1576–1582
Takano-Ohmuro H, Mukaida M, Kominami E, Morioka K (2000) Autophagy in embryonic erythroid cells: its role in maturation. Eur J Cell Biol 79(10):759–764
Zhang J, Ney PA (2009) Autophagy-dependent and -independent mechanisms of mitochondrial clearance during reticulocyte maturation. Autophagy 5(7):1064–1065
Zhang J, Randall MS, Loyd MR, Dorsey FC, Kundu M, Cleveland JL, Ney PA (2009) Mitochondrial clearance is regulated by atg7-dependent and -independent mechanisms during reticulocyte maturation. Blood 114(1):157–164. doi:10.1182/blood-2008-04-151639
Sripichai O, Makarasara W, Munkongdee T, Kumkhaek C, Nuchprayoon I, Chuansumrit A, Chuncharunee S, Chantrakoon N, Boonmongkol P, Winichagoon P, Fucharoen S (2008) A scoring system for the classification of beta-thalassemia/hb E disease severity. Am J Hematol 83(6):482–484
Langlois S, Ford JC, Chitayat D, Desilets VA, Farrell SA, Geraghty M, Nelson T, Nikkel SM, Shugar A, Skidmore D, Allen VM, Audibert F, Blight C, Gagnon A, Johnson JA, Wilson RD, Wyatt P (2008) Carrier screening for thalassemia and hemoglobinopathies in Canada. J Obstet Gynaecol Can 30(10):950–971
Chong SS, Boehm CD, Higgs DR, Cutting GR (2000) Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood 95(1):360–362
Dode C, Krishnamoorthy R, Lamb J, Rochette J (1993) Rapid analysis of -alpha 3.7 thalassaemia and alpha alpha alpha anti 3.7 triplication by enzymatic amplification analysis. Br J Haematol 83(1):105–111
McGrath KE, Bushnell TP, Palis J (2008) Multispectral imaging of hematopoietic cells: where flow meets morphology. J Immunol Methods 336(2):91–97
Panyasrivanit M, Khakpoor A, Wikan N, Smith DR (2009) Co-localization of constituents of the dengue virus translation and replication machinery with amphisomes. J Gen Virol 90(Pt 2):448–456. doi:10.1099/vir.0.005355-0
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with ImageJ. Biophoton Int 11:36–42
French AP, Mills S, Swarup R, Bennett MJ, Pridmore TP (2008) Colocalization of fluorescent markers in confocal microscope images of plant cells. Nat Protoc 3(4):619–628. doi:10.1038/nprot.2008.31
Mathias LA, Fisher TC, Zeng L, Meiselman HJ, Weinberg KI, Hiti AL, Malik P (2000) Ineffective erythropoiesis in beta-thalassemia major is due to apoptosis at the polychromatophilic normoblast stage. Exp Hematol 28(12):1343–1353
Glick D, Barth S, Macleod KF (2010) Autophagy: cellular and molecular mechanisms. J Pathol 221(1):3–12. doi:10.1002/path.2697
Wickramasinghe SN, Lee MJ (1998) Evidence that the ubiquitin proteolytic pathway is involved in the degradation of precipitated globin chains in thalassaemia. Br J Haematol 101(2):245–250
Schrier SL (2002) Pathophysiology of thalassemia. Curr Opin Hematol 9(2):123–126
Yousefi S, Simon HU (2007) Apoptosis regulation by autophagy gene 5. Crit Rev Oncol Hematol 63(3):241–244. doi:10.1016/j.critrevonc.2007.06.005
Pootrakul P, Sirankapracha P, Hemsorach S, Moungsub W, Kumbunlue R, Piangitjagum A, Wasi P, Ma L, Schrier SL (2000) A correlation of erythrokinetics, ineffective erythropoiesis, and erythroid precursor apoptosis in Thai patients with thalassemia. Blood 96(7):2606–2612
Acknowledgments
This work was supported by a Research Strengthening Grant from the National Center for Genetic Engineering and Biotechnology (BIOTEC), National Science and Development Agency (NSTDA) and Mahidol University. P.L. is supported by a Thai Royal Golden Jubilee Research Ph.D. Scholarship, A.L. is supported by the Thai Commission on Higher Education Ph.D. Scholarship, and T.W was supported by a BIOTEC Ph.D. Scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lithanatudom, P., Wannatung, T., Leecharoenkiat, A. et al. Enhanced activation of autophagy in β-thalassemia/Hb E erythroblasts during erythropoiesis. Ann Hematol 90, 747–758 (2011). https://doi.org/10.1007/s00277-010-1152-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-1152-5